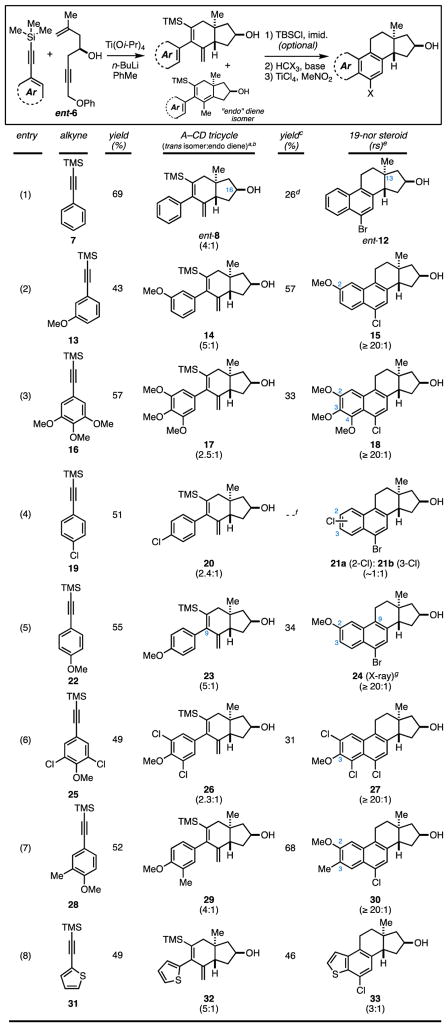
Table 1.
yield reported is for the combination of diene iosmers (trans + endo).
stereoselectivity for the annulation process (trans:cis) is typically ~6:1
yield reported is for the 2–3 step sequence.
cyclization sequence was conducted without protection of the C16 hdyroxy group.
regioselectivity based on 1H NMR of the crude reaction product.
yield for this unselective process was not determined.
structure confirmed by X-ray diffraction - See Supporting Information.