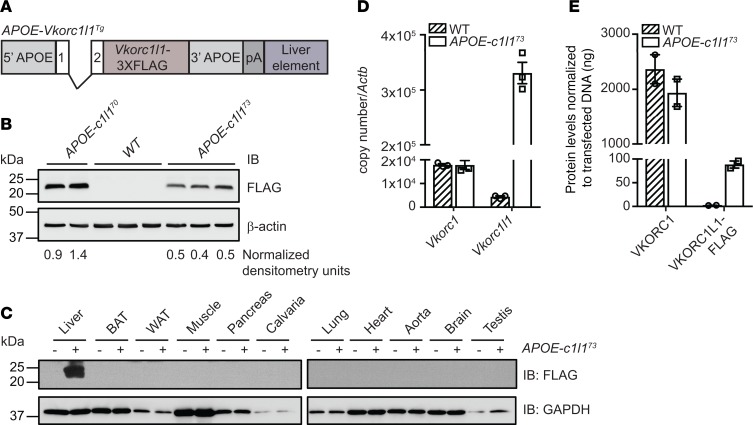
Figure 5. Generation and characterization of APOE-Vkorc1l1 transgenic mice.
(A) Schematic representation of the construct used to generate APOE-Vkorc1l1Tg (APOE-c1l1Tg) mice. Vkorc1l1-3XFLAG was cloned in the pLIV.7 vector containing exon 1 and 2, liver-specific regulatory sequences and 5′ and 3′ flanking sequences of the human APOE gene. (B) VKORC1L1-FLAG expression in livers from WT, APOE-Vkorc1l170 (APOE-c1l170), and APOE-Vkorc1l173 (APOE-c1l173) transgenic mice at 4 weeks of age was assessed by Western blot analysis using α-FLAG antibody. β-Actin was used as a loading control. β-Actin–normalized arbitrary densitometry units of VKORC1L1-FLAG are shown. (C) VKORC1L1-FLAG expression in tissues from WT and APOE-Vkorc1l173 mice was detected by Western blot analysis with α-FLAG antibody. GAPDH was used as a loading control. (D) Gene expression level of Vkorc1 and Vkorc1l1 in livers from WT and APOE-Vkorc1l173 mice at 4 weeks of age was assessed by qPCR and normalized to Actb (n = 3; mean ± SEM). Copy number was quantified using plasmid DNA containing either Vkorc1 or Vkorc1l1 cDNA as a standard. (E) VKORC1 and VKORC1L1-FLAG protein levels in liver from WT and APOE-Vkorc1l173 mice were measured by Western blot using α-VKORC1 and α-FLAG antibodies. VKORC1-FLAG– and VKORC1L1-FLAG–transfected HEK293 cells were used as a standard and β-actin as a loading control (n = 2; mean ± SEM; Supplemental Figure 4).