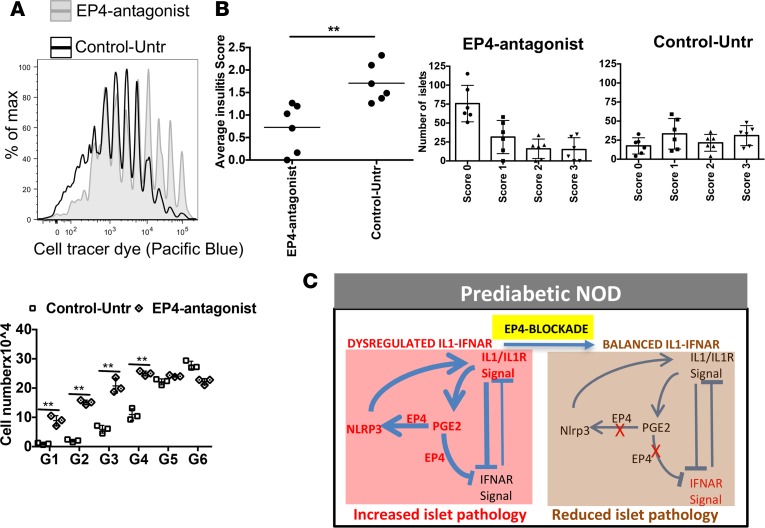
Figure 7. In vivo EP4 antagonist treatment decreases autoimmune pathology.
(A) NOD mice treated with grapiprant (EP4 antagonist) or untreated (control) for 4 weeks were then injected with cell tracer dye–labeled transgenic BDC2.5 CD4 T cells, and cell proliferation was checked 6 days after transfer. Cell division was compared between EP4 antagonist–treated and untreated control mice by counting cell numbers for each division. (B) Separate group of control or EP4-inhibited mice (did not received BDC2.5 CD4 T cells) were checked for islet lymphocyte infiltration (6 weeks after treatment). Pancreas tissue samples were sectioned for H&E staining, and islet infiltration was ranked as follows: no infiltration, 0; 10%–20%, 1; 20%–50%, 2; and >50%, 3. Data are presented with average score or number of islets with different levels of infiltration with lymphocytes. Data are representative of 2 independent experiments (A and B). Each experiment was performed with 3–4 mice per group. The graph shows mean ± SD for each of the experiments. Statistical analysis was performed with 1-way ANOVA with Bonferroni post-test. (C) Proposed schematic model of altered balance of IL-R/IL-1 and IFNAR signaling in adult prediabetic NOD mice with modulation by PGE2-EP4 and NLRP3, and reestablishment of homeostatic balance after inhibiting EP4 signals. Prediabetic NOD mice showed increased expression of IL1, PGE2, PTGER4, and NLRP3 (red). PGE2-EP4 were key regulators of enhancing NLRP3 and IL1 that was inhibitory to IFNAR signaling (bold lines). After inhibition of EP4, IFNAR signaling increased by reducing IL1.