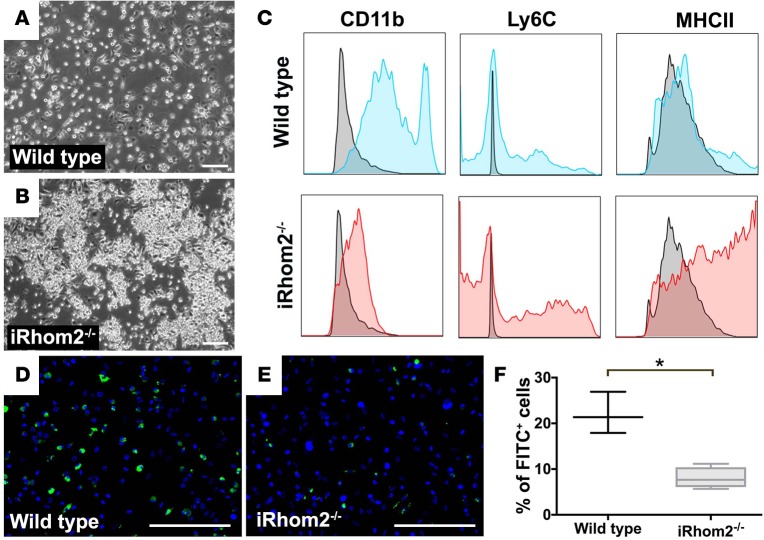
Figure 7. Immunophenotyping of BMDMs from iRhom2-deficient mice.
Bone marrow cells were differentiated in conditioned media for 7 days to generate bone marrow–derived macrophages (BMDMs). Bright-field images revealed (A) wild-type cultures form a dispersed monolayer of cells, (B) while iRhom2-deficient (iRhom2–/–) BMDM cultures formed clusters of cell aggregates (arrowheads). (C) Flow cytometry shows F4/80+ macrophages expressed moderate to high levels of the myeloid differentiation maker CD11b (MFI: 42,758 ± 2,955). However, F4/80+ iRhom2–/– cells expressed low levels of CD11b (MFI: 1,266 ± 64) and increased expression of the monocyte marker Ly6C (MFI: 10,631 ± 716), as well as an increased expression in the late-stage monocyte marker class II MHC (MFI: 34,324 ± 3,687), as compared with wild-type BMDMs (Ly6C MFI: 3,198 ± 1,150; MHCII MFI: 12,733 ± 3,814). (D–F) Phagocytosis assays revealed iRhom2-deficient BMDMs were functionally impaired and failed to phagocytose FITC-labeled latex beads as efficiently as control BMDMs. Scale bars: 200 μm. n = 3 for each experimental group. Data are shown as the mean ± SEM, *P ≤ 0.05, Student’s t test. MFI, mean fluorescence intensity.