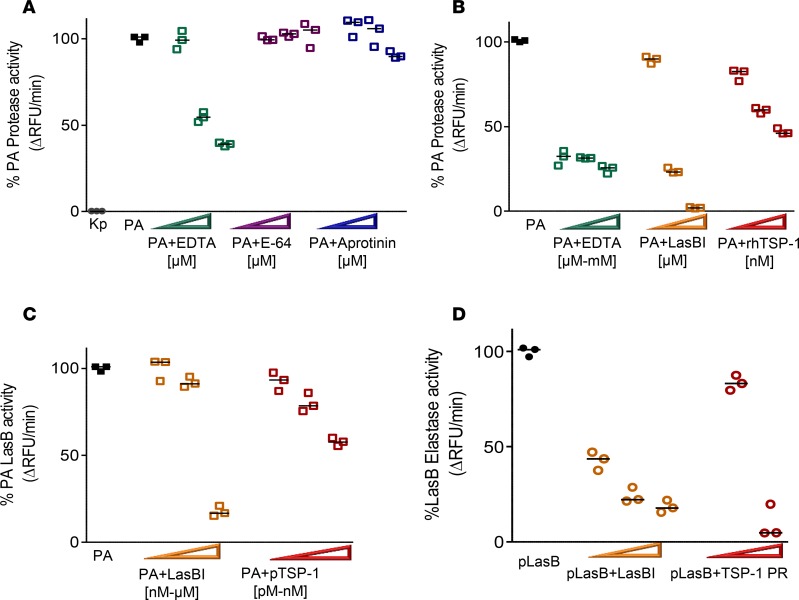
Figure 5. The majority of protease activity secreted by Pseudomonas aeruginosa (PA) exhibits the profile of a metalloprotease and TSP-1 shows dose-dependent inhibition of PA exoprotease activity.
(A) PA cell-free supernatant (SN) was tested for total protease activity by measuring the hydrolysis of fluorogenic casein substrate over time in the presence or absence of inhibitor. Increasing width of arrowheads indicates increasing molar concentrations of inhibitors. Although SN from Klebsiella pneumoniae (Kp) grown under the same conditions shows no detectable protease activity, that from PA exhibits robust protease activity that is inhibited by EDTA (20, 50, and 100 μM). The effects of serine protease inhibitor aprotinin (20, 50, and 100 μM), and cysteine protease inhibitor E-64 (20, 50, and 100 μM) on PA protease activity are shown for comparison. (B) Recombinant human TSP-1 (rhTSP-1) dose-dependently inhibits PA protease activity (rhTSP-1 at 78, 156, and 312 nM). This is compared with the inhibitory activity of EDTA (50 μM, 500 μM, and 5 mM), and specific LasB inhibitor (LasBI) N-mercaptoacetyl-Phe-Tyr-amide (1, 10, and 100 μM). PA SN in the absence of inhibitor is the reference control set as 100% protease activity. (C) Human purified TSP-1 (200 pM, 1 nM, and 78 nM) dose-dependently inhibits PA LasB activity as measured by hydrolysis of specific LasB substrate aminobenzoyl-Ala-Gly-Leu-Ala-p-nitro-benzyl-amide. This is compared with the inhibitory activity of LasBI (78 nM, 312 nM, and 20 μM). (D) Purified LasB protein (pLasB) was incubated with human TSP-1 from thrombin-activated platelet releasates (PRs) at 1 μg or 10 μg total protein concentrations and elastase activity measured by hydrolysis of elastin substrate over time. LasBI (500 nM, 50 μM, and 100 μM) was incubated with pLasB protein and the degree of percentage pLasB elastase activity inhibition is shown as a comparison. (A–D) Assays were performed in triplicate and a representative study of 3 independent experiments is shown. Lines indicate the median.