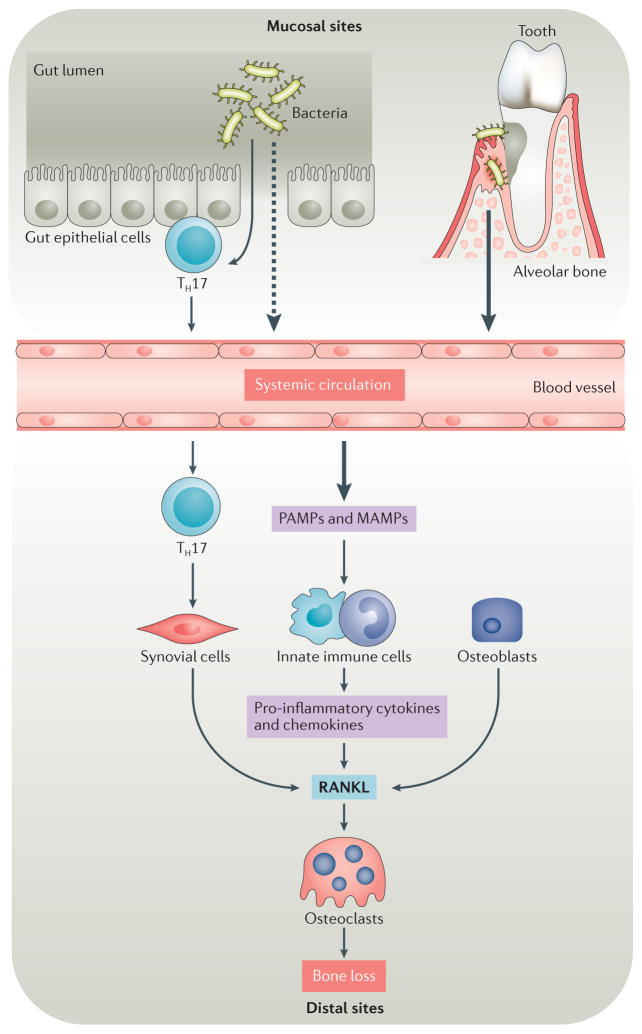
Figure 2. Microbiota and osteoimmunology.
Under homeostatic or inflammatory conditions, microbiota can affect bone resorption at sites proximal to and distal to the site of colonization. Inflammatory conditions are often triggered by dysbiosis of microbiota, which can be associated with the outgrowth of particular phyla and species. Molecular triggers from gut microbiota cross the epithelial boundary more readily under dysbiotic and/or inflammatory conditions than during homeostasis and might eventually influence bone at distal sites, either by entering systemic circulation or by activating local adaptive immune elements, such as T helper 17 (TH17) cells within the mucosal tissue that can transit to distal sites and therein exert their effector function. By contrast, stimuli from microbiota in the subgingival crest between the tooth and the gum act proximally on the alveolar bone that anchors teeth. Oral microbiota can also contribute to inflammatory bone loss at distal sites, such as the joints of patients with rheumatoid arthritis. Pathogen-associated molecular patterns (PAMPs) and microbiota-associated molecular patterns (MAMPs) trigger signalling pathways in innate immune cells and synovial fibroblasts that induce the production of pro-inflammatory cytokines and chemokines. These factors contribute to increased expression of receptor activator of nuclear factor-κB ligand (RANKL) and increased osteoclast differentiation and activation, which increase inflammatory bone loss at both distal sites and proximal sites.