Abstract
Objective
To assess the impact of discontinuing oral hormone therapy (HT) on sexual activity, vaginal symptoms, and sexual activity components among participants in the Estrogen-Progestin Therapy (EPT) and Estrogen Therapy (ET) trial of the Women’s Health Initiative.
Methods
Surveys were sent postintervention to those who were still taking study pills and agreed to continue in the study when the trials were stopped. Comparisons between former HT and placebo users were accomplished with chi-square for categorical variables and t-tests for continuous variables.
Results
13,902 women with mean age at survey 69.9 years (EPT trial, women with intact uterus) and 71.7 years (ET trial, women with history of hysterectomy) responded. Prevalence of sexual activity postintervention was not significantly different between former EPT and placebo users (36.0% vs 34.2%, P=0.37). Sexual activity of former ET users was 5.6% higher than placebo users (27.6% vs 22.0%, P=0.001). The majority of sexually active women overall maintained orgasmic capacity and sexual satisfaction. Former EPT users were 10%–12% more likely than former placebo users to report decreased desire, arousal, intercourse, climax, and satisfaction with sexual activity as well as increased dryness and dyspareunia upon discontinuing study drugs (P<0.001). Former ET users were more likely than placebo users to report rare to no desire or arousal postintervention (P<0.001).
Conclusions
Postintervention ET trial participants formerly assigned to ET were significantly more likely to report sexual activity than those formerly assigned to placebo. Women who discontinued EPT were significantly more likely to report negative vaginal and sex-related effects.
Keywords: hormone therapy discontinuation, sexual function, genitourinary syndrome of menopause
Introduction
Many obstacles to sexual activity are encountered with aging. In addition to loss of partner and medical conditions, low levels of estrogen associated with menopause are considered to play an important role in postmenopausal female sexual function.1–3 Low estrogen levels lead to physiologic changes in the vulva and vagina that may result in symptoms of the genitourinary syndrome of menopause (GSM).4 Common sexual complaints associated with GSM include vaginal dryness, irritation, burning, and dyspareunia. These symptoms can have an adverse effect on sexual activity. The syndrome also includes lower urinary tract symptoms of dysuria, urgency and recurrent urinary tract infections.
Hormone therapy (HT), both systemic and local, alleviates vulvovaginal symptoms of GSM, but data are sparse regarding the effects of discontinuing HT on vaginal symptoms and sexual function. Reports have focused primarily on vaginal dryness.5–9 To assess in greater detail the impact of discontinuing oral HT on sexual activity and vaginal symptoms in older postmenopausal women, we analyzed postintervention phase data from participants in the Women’s Health Initiative (WHI) Estrogen-progestin therapy (EPT) and Estrogen therapy (ET) trials whose mean ages were 70 and 72 years, respectively, at the time study pills were discontinued.
Methods
The design and primary outcomes of the two WHI HT trials have been described and updated extensively.10–13 Briefly, the HT trials comprised 27,347 postmenopausal women aged 50 to 79 years at baseline enrolled at 40 centers across the United States into the EPT trial or, for those without a uterus, the ET trial. EPT consisted of daily oral conjugated equine estrogens 0.625 mg plus oral 2.5 mg medroxyprogesterone acetate, and ET consisted of daily oral conjugated equine estrogens 0.625 mg. Although women with vasomotor symptoms after hormone therapy washout were discouraged from participating, symptoms of GSM were not included in enrollment screening questions nor were they specified as exclusion criteria.
The EPT intervention was discontinued July 7, 2002, and the ET intervention February 29, 2004. Participants were notified by letter to discontinue their study pills. Both trials were discontinued early (after a median duration of 5.6 and 7.2 years, respectively) based on data and safety monitoring board conclusions that benefits did not outweigh the adverse events and because a further increase in benefit was not expected. Within three months after the letter mailing, women were informed of their randomization assignment in-person or by telephone, at which time it was confirmed that they had discontinued their study pills. All participants in the HT trials completed extensive questionnaires at baseline. A brief and limited instrument was used to assess sexual activity14 during the intervention phase at baseline and at one year after randomization by all HT participants and then again at years 3 and 6 by an 8.6% subsample.
Derivation of the cohort for this report is illustrated in Figure 1. New sexual activity questions were developed for a postintervention survey that was mailed to participants who agreed to continue in the study and were still taking study pills at the time the trials were stopped. The sexual activity questions in the postintervention survey for both HT trials were similar, but not identical. Seven key clinical characteristics of sexual activity – frequency of intercourse, discomfort with intercourse, desire, arousal, ability to climax, vaginal tightness, and satisfaction with sexual activity – were included in both surveys, but the response options were different. The options in the EPT survey compared current symptoms with previous symptoms (less, greater, the same) while the options in the ET survey were related to current symptom frequency (a lot of the time, some of the time, rarely or not at all).
FIG. 1.
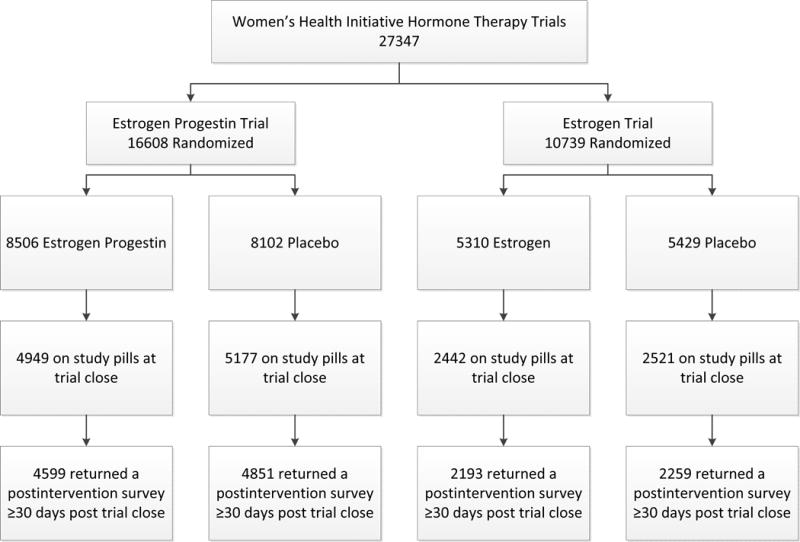
Study CONSORT flow diagram.
Figures 2A and 2B summarize in a condensed format the questions related to sexual activity in the two distinct questionnaires. A long list of physical symptoms that included common vulvovaginal symptoms was administered routinely, as indicated above, during the active phase of the study, as well as postintervention. The list included only one of the three GSM urinary tract symptoms, dysuria, which was represented in the questionnaire as pain or burning while urinating. The timeframe for symptom occurrence on these items was the last 4 weeks. Dyspareunia, represented as discomfort with intercourse, was included only in the postintervention survey. The majority (92%) of surveys returned were received between 8 and 13 months after each trial stopped. No pelvic examination was conducted postintervention.
FIG. 2.(A).
Estrogen Plus Progestin Trial Survey questions related to sexual activity.
FIG. 2.(B).
Estrogen Trial Survey questions related to sexual activity.
Basic demographics such as ethnicity, education and income level were assessed at initial enrollment into the study. Body mass index (BMI; assessed annually), other medical history information (assessed semi-annually), and partner status or living with partner were obtained from the visit most proximal to the postintervention survey mailing.
Statistical analysis
Demographic characteristics and medical conditions of respondents who were and were not sexually active were described using frequencies and percentages for categorical variables and means and standard deviations for continuous variables. Comparisons of these characteristics were carried out using either chi-square for categorical variables or t-tests for continuous variables.
Age and partner status of participants were compared by intervention arm using chi-square or t-tests. For the sexual activity status questions, women who were sexually active vs. inactive were compared using logistic regression modeling, with sexual activity as a function of intervention arm and adjusted for sexual activity at WHI enrollment baseline.
Responses to the sexual activity question by age group and partner status (living with a partner) at time of the survey were described with frequencies and percentages. P-values for linear trend of sexual activity vs. inactivity by age groups were generated using logistic regression models. Characteristics of postintervention sexual activity by intervention arm were described with frequencies and percentages and compared using Chi-square analyses for each question. P-values for linear trends in sexual activity components across age groups were calculated, with the sexual variable of interest as a function of ET intervention arm, linear age group, and their interaction.
Results
A total of 13,902 women responded to postintervention questionnaires, 9450 in the EPT trial and 4452 in the ET trial, representing a response rate of 93% and 90%, respectively (Fig.1). Characteristics of the respondents from the two trials are presented in Table 1. Demographic and body mass index data presented in Table 2 by sexual activity suggest that higher education, higher income, and lower body mass index are associated with sexual activity. There were no significant racial/ethnic differences in sexual activity postintervention. Women who were sexually inactive were significantly more likely than sexually active women to report a history of medical conditions such as cancer, myocardial infarction, congestive heart failure, diabetes mellitus, hyperlipidemia, hypertension, arthritis, depression, and bilateral oophorectomy (Table 3). Sexually inactive women were more than twice as likely to report poor to fair self-rated health compared with sexually active women (p<0.001).
TABLE 1.
| EPT
Trial n=9450 |
ET
Trial n=4452 |
|||
|---|---|---|---|---|
| n | % | n | % | |
| Age (yrs) at postintervention survey, mean (SD) | 69.9 (6.7) | 71.7 (6.9) | ||
| Ethnicity | ||||
| White | 8135 | 86.1 | 3473 | 78.0 |
| Black | 529 | 5.6 | 587 | 13.2 |
| Hispanic | 434 | 4.6 | 228 | 5.1 |
| American Indian | 27 | 0.3 | 29 | 0.7 |
| Asian/Pacific Islander | 209 | 2.2 | 72 | 1.6 |
| Unknown | 116 | 1.2 | 63 | 1.4 |
| Education | ||||
| Less than high school diploma/GED | 515 | 5.4 | 343 | 7.7 |
| High school diploma/GED | 1879 | 19.9 | 1004 | 22.6 |
| School after high school | 3575 | 37.8 | 1905 | 42.8 |
| College degree or higher | 3431 | 36.3 | 1162 | 26.1 |
| Income | ||||
| < $20,000 | 1622 | 17.2 | 1056 | 23.7 |
| $20,000 – $74,999 | 6091 | 64.5 | 2808 | 63.1 |
| $75,000 + | 1264 | 13.4 | 387 | 8.7 |
| Body mass index, kg/m2 | ||||
| < 25 | 2649 | 28.0 | 907 | 20.4 |
| 25 – 29 | 3361 | 35.6 | 1508 | 33.9 |
| 30 – 34 | 2085 | 22.1 | 1144 | 25.7 |
| ≥ 35 | 1355 | 14.3 | 893 | 20.1 |
| History of cancer | 392 | 4.1 | 310 | 7.0 |
| History of myocardial infarction | 235 | 2.5 | 167 | 3.8 |
| History of congestive heart failure | 122 | 1.3 | 103 | 2.3 |
| History of diabetes mellitus | 820 | 8.7 | 720 | 16.2 |
| History of hyperlipidemia | 2185 | 23.1 | 1478 | 33.2 |
| History of hypertension | 4241 | 44.9 | 2723 | 61.2 |
| History of arthritis | 4879 | 51.6 | 2801 | 62.9 |
| History moderate/severe urine leakage | 3323 | 35.2 | 1977 | 44.4 |
| Depression construct (0–18), mean (SD)c | 2.4 (2.5) | 2.4 (2.6) | ||
| History of antidepressant use | 952 | 10.1 | 647 | 14.5 |
| Poor to fair self-rated health | 516 | 5.5 | 642 | 14.4 |
| Self-reported quality of life (0–10), mean (SD)d | 8.0 (1.6) | 8.0 (1.6) | ||
Demographic data obtained at original Women’s Health Initiative study enrollment baseline.
BMI data and medical conditions obtained from most recent assessment.
Higher score indicates greater depression.
Higher score indicates better quality of life.
EPT, estrogen-progestin therapy.
ET, estrogen therapy.
SD, standard deviation.
GED, General Education Development.
TABLE 2.
Sexual activity postintervention reported by Hormone Therapy trial participants, by demographics and body mass indexa,b
| Sexually activec | Sexually inactive | Did not respond | |||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | P | |
| All Participants | 4414 | 31.8 | 7356 | 52.9 | 2132 | 15.3 | |
| Age (yrs) at original WHI study enrollment baseline, mean (SD) | 4414 | 60.7 | 7356 | 64.2 | 2132 | 63.6 | <0.001 |
| Age (yrs) at postintervention survey, mean (SD) | 4414 | 68.1 | 7356 | 71.7 | 2132 | 71.2 | <0.001 |
| Ethnicity | 0.11 | ||||||
| White | 3789 | 32.6 | 6198 | 53.4 | 1621 | 14.0 | |
| Black | 298 | 26.7 | 556 | 49.8 | 262 | 23.5 | |
| Hispanic | 191 | 28.9 | 350 | 52.9 | 121 | 18.3 | |
| American Indian | 11 | 19.6 | 37 | 66.1 | 8 | 14.3 | |
| Asian/Pacific Islander | 75 | 26.7 | 127 | 45.2 | 79 | 28.1 | |
| Unknown | 50 | 27.9 | 88 | 49.2 | 41 | 22.9 | |
| Education | <0.001 | ||||||
| Less than high school diploma/GED | 173 | 20.2 | 490 | 57.1 | 195 | 22.7 | |
| High school diploma/GED | 787 | 27.3 | 1604 | 55.6 | 492 | 17.1 | |
| School after high school | 1734 | 31.6 | 2909 | 53.1 | 837 | 15.3 | |
| College degree or higher | 1691 | 36.8 | 2304 | 50.2 | 598 | 13.0 | |
| Income | <0.001 | ||||||
| < $20,000 | 506 | 18.9 | 1706 | 63.7 | 466 | 17.4 | |
| $20,000 – $74,999 | 2893 | 32.5 | 4692 | 52.7 | 1314 | 14.8 | |
| $75,000 + | 842 | 51.0 | 614 | 37.2 | 195 | 11.8 | |
| Sexual orientation | <0.001 | ||||||
| Never had Sex | 4 | 2.1 | 144 | 74.2 | 46 | 23.7 | |
| Lifetime/mature lesbian | 26 | 37.7 | 34 | 49.3 | 9 | 13.0 | |
| Heterosexual | 4210 | 32.8 | 6762 | 52.6 | 1880 | 14.6 | |
| Bisexual | 40 | 47.6 | 37 | 44.0 | 7 | 8.3 | |
| Prefer not to answer | 28 | 8.8 | 173 | 54.1 | 119 | 37.2 | |
| Body mass index (BMI), kg/m2 | <0.001 | ||||||
| < 25 | 1177 | 33.1 | 1876 | 52.8 | 503 | 14.1 | |
| 25 – 29 | 1625 | 33.4 | 2486 | 51.1 | 758 | 15.6 | |
| 30 – 34 | 991 | 30.7 | 1741 | 53.9 | 497 | 15.4 | |
| ≥ 35 | 621 | 27.6 | 1253 | 55.7 | 374 | 16.6 | |
Demographic data obtained at original WHI study enrollment baseline.
BMI data obtained from most recent assessment.
Sexually active includes activity with a partner or self-stimulation. Estrogen-Progestin Therapy trial participants were asked “Has sexual activity been part of your life since you stopped study pills?”; Estrogen Therapy trial participants were asked “Has sexual activity been part of your life in the last 3 months?”
P value compares sexually active vs. inactive.
WHI, Women’s Health Initiative.
GED, General Education Development.
TABLE 3.
Medical conditions reported by Hormone Therapy trial participants postintervention, by sexual activity statusa
| Sexually activeb n =4414 |
Sexually
inactive n=7356 |
Did not
respond n=2132 |
|||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | Pc | |
| History of cancer | 187 | 4.2 | 404 | 5.5 | 111 | 5.2 | 0.003 |
| History of myocardial infarction | 101 | 2.3 | 232 | 3.2 | 69 | 3.2 | 0.006 |
| History of congestive heart failure | 47 | 1.1 | 133 | 1.8 | 45 | 2.1 | 0.001 |
| History of diabetes mellitus | 384 | 8.7 | 866 | 11.8 | 290 | 13.6 | <0.001 |
| History of hyperlipidemia | 1007 | 22.8 | 2037 | 27.7 | 619 | 29.0 | <0.001 |
| History of hypertension | 1953 | 44.2 | 3874 | 52.7 | 1137 | 53.3 | <0.001 |
| History of arthritis | 2208 | 50.0 | 4286 | 58.3 | 1186 | 55.6 | <0.001 |
| History of hysterectomy | 1173 | 26.6 | 2591 | 35.2 | 903 | 42.4 | <0.001 |
| History of bilateral oophorectomy | 402 | 9.1 | 1017 | 13.8 | 313 | 14.7 | <0.001 |
| History moderate/severe urine leakage | 1576 | 35.7 | 2975 | 40.4 | 749 | 35.1 | <0.001 |
| History of antidepressant use | 416 | 9.4 | 957 | 13.0 | 226 | 10.6 | <0.001 |
| Depression construct (0–18), mean (SD)d | 2.1 (2.3) | 2.5 (2.7) | 2.5 (2.6) | <0.001 | |||
| Poor to fair self-rated health | 198 | 4.5 | 731 | 9.9 | 229 | 10.7 | <0.001 |
| Quality of life (0–10), mean (SD)e | 8.3 (1.5) | 7.9 (1.7) | 8.0 (1.7) | <0.001 | |||
Based on most recent assessment with exception of bilateral oophorectomy which was assessed at original Women’s Health Initiative study enrollment baseline.
Sexually active includes activity with a partner or self-stimulation. Estrogen-Progestin Therapy trial participants were asked “Has sexual activity been part of your life since you stopped study pills”; Estrogen Therapy trial participants were asked “Has sexual activity been part of your life in the last 3 months?”
P value compares sexually active vs. inactive.
Higher score indicates greater depression.
Higher score indicates better quality of life.
SD, standard deviation.
Sexual activity, either with a partner or by self-stimulation, is reported in Table 4 according to HT trial participation and intervention arm. The percentage of participants with a partner was similar between active and placebo arms within each trial. Slightly more EPT than ET respondents reported being partnered at both the original WHI enrollment baseline (65% vs. 61%) and postintervention (55% vs. 51%). When the studies were closed out, 67% of participants in this cohort answered a question about satisfaction with their frequency of sexual activity. Of those, approximately 25% indicated they preferred more sexual activity and 5% preferred less, with the remainder indicating they were satisfied with their current frequency. The responses were very similar across trials and intervention arms (data not shown).
TABLE 4.
Age, partner status, and sexual activity at original WHI study enrollment baseline and at postintervention, by trial participation and intervention arm
| Estrogen-Progestin Therapy (EPT) | Estrogen Therapy (ET) Trial | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| EPT (n=4599) |
Placebo (n=4851) |
P | ET (n=2193) |
Placebo (n=2259) |
P | |||||
| n | % | N | % | n | % | n | % | |||
| Age (yrs) at WHI baseline, mean (SD) | 62.6 (6.9) | 63.3 (6.9) | <0.001 | 62.8 (7.0) | 63.4 (7.2) | 0.01 | ||||
| Age (yrs) at survey, mean (SD) | 69.6 (6.8) | 70.2 (6.7) | <0.001 | 71.4 (6.8) | 72.0 (7.1) | 0.01 | ||||
| Days from trial close to survey, mean (SD) | 304.4 (43.2) | 305.5 (44.7) | 0.22 | 302.3 (54.9) | 300.5 (55.6) | 0.26 | ||||
| Partnered at baseline | 3017 | 65.6 | 3141 | 64.7 | 0.68 | 1369 | 62.4 | 1365 | 60.4 | 0.39 |
| Partnered at surveya | 2564 | 55.8 | 2627 | 54.2 | 0.17 | 1142 | 52.1 | 1120 | 49.6 | 0.10 |
| Sexual activity with a partner at baseline | 0.05b | 0.05b | ||||||||
| Yes | 2342 | 50.9 | 2362 | 48.7 | 1053 | 48.0 | 1002 | 44.4 | ||
| No | 2113 | 45.9 | 2311 | 47.6 | 1056 | 48.2 | 1168 | 51.7 | ||
| No response | 144 | 3.1 | 178 | 3.7 | 84 | 3.8 | 89 | 3.9 | ||
| Sex activity since discontinuing study pills (EPT Trial) or in past 3 months (ET Trial) among those with a partner | 0.48c | 0.002c | ||||||||
| Yesd | 1357 | 52.9 | 1346 | 51.2 | 507 | 44.4 | 413 | 36.9 | ||
| No | 866 | 33.8 | 907 | 34.5 | 407 | 35.6 | 458 | 40.9 | ||
| No response | 341 | 13.3 | 374 | 14.2 | 228 | 20.0 | 249 | 22.2 | ||
| Sex activity overall since discontinuing study pills (EPT Trial) or in past 3 months (ET Trial) | 0.37c | 0.001c | ||||||||
| Yesd | 1654 | 36.0 | 1658 | 34.2 | 606 | 27.6 | 496 | 22.0 | ||
| No | 2342 | 50.9 | 2533 | 52.2 | 1172 | 53.4 | 1309 | 57.9 | ||
| No response | 603 | 13.1 | 660 | 13.6 | 415 | 18.9 | 454 | 20.1 | ||
Median time from partner status variable is 591 days post-survey for EPT trial and 0 days for ET trial.
P-value from a Chi-square test comparing sexual activity (yes vs. no) by intervention arm.
P-value from a logistic regression model with sexual activity (yes vs. no) as a function of intervention arm, adjusted for sexual activity with a partner at baseline (categorical: yes, no, no response).
Includes sexual activity with a partner or self-stimulation.
WHI, Women’s Health Initiative.
SD, standard deviation.
Sexual activity since stopping pills (a mean of 10 months after EPT trial closure) was reported by approximately 35% of EPT respondents overall and by 52% of those who were partnered (Table 4). In the EPT trial there was no statistically significant difference between those formerly assigned to EPT and those formerly assigned to placebo in the percentage who were sexually active postintervention.
A mean of 10 months after ET trial closure, respondents were asked if they experienced sexual activity in the last 3 months. Approximately 25% of ET respondents overall and 41% of those who were partnered replied affirmatively. Despite a lower rate of being partnered and a lower prevalence of sexual activity, 5.6% more women formerly assigned to ET than placebo reported sexual activity in the last 3 months (p<0.001). Among those with a partner, 7.5% more women formerly assigned to ET than placebo were sexually active (p<0.002).
Analysis of the ET trial results by oophorectomy status revealed that only women who reported bilateral oophorectomy showed a statistically significant difference in the prevalence of sexual activity between those formerly assigned to ET and those formerly assigned to placebo, 27.4% and 19.0%, respectively (p<0.001). Among ET participants reporting the presence of one or two ovaries, there was no statistically significant difference in the prevalence of sexual activity (Table 5).
TABLE 5.
Sexual activity in past three months reported by postintervention respondents based on number of ovaries present, by Estrogen Therapy trial intervention arma,b
| Estrogen Therapy Trial | |||||
|---|---|---|---|---|---|
| Estrogen (n=2193) |
Placebo (n=2259) |
Pb | |||
| n | % | n | % | ||
| Women with 2 ovaries | (n=941) | (n=915) | 0.16 | ||
| Sexual activity reported | 271 | 28.8 | 234 | 25.6 | |
| No sexual activity reported | 500 | 53.1 | 501 | 54.8 | |
| No response | 170 | 18.1 | 180 | 19.7 | |
| Women with 1 ovary | (n=274) | (n=289) | 0.41 | ||
| Sexual activity reported | 76 | 27.8 | 65 | 22.5 | |
| No sexual activity reported | 131 | 47.8 | 153 | 52.9 | |
| No response | 67 | 24.5 | 71 | 24.6 | |
| Women with bilateral oophorectomy | (n=815) | (n=890) | <0.001 | ||
| Sexual activity reported | 223 | 27.4 | 169 | 19.0 | |
| No sexual activity reported | 449 | 55.1 | 555 | 62.4 | |
| No response | 143 | 17.5 | 166 | 18.7 | |
Includes sexual activity with a partner or self-stimulation.
Number of ovaries was assessed at original Women’s Health Initiative study enrollment baseline.
P value from a logistic regression model with sexual activity (yes vs. no) as a function of intervention arm, adjusted for sexual activity with a partner at baseline (categorical: yes, no, no response).
From 51% to 58% of all women in the cohort reported no sexual activity since discontinuing study pills (EPT) or in the last 3 months (ET). The chief reason for no sexual activity given by almost half of these women was lack of partner. Other reasons for no sexual activity in descending order of frequency included woman not interested, partner not able, partner not interested, other, and woman not able (Fig. 3). The various reasons were ranked in the same order in both HT trials.
FIG. 3.

Reasons women were not sexually active reported as a percentage of all respondents from both hormone trials who indicated no sexual activity postintervention (n=7356). Respondents were instructed to mark all that apply.
Across the age groups overall there was a decline in sexual activity from 46% among women aged less than 60 years of age to 9% among those aged over 80 years (p-value for trend <0.001) (Table 6). Among women with a partner, the corresponding distribution of sexual activity by age was 62% among those less than 60 years of age and 25% among those over age 80 years, not including report of self-stimulation only. Only 31% of women age 80 years and older had a partner. The report of sexual activity through self-stimulation only was higher among women without partners across all age groups, with the rate highest (11%) among women under age 60 and decreasing to 6% among women over age 80. Considering the entire cohort, the rate of sexual activity by self-stimulation only remained fairly constant across all age groups at ~5%.
TABLE 6.
Postintervention sexual activity with partner or by self-stimulation in Hormone Therapy trial participants, by age group postintervention
| Total | <60 | 60–69 | 70–79 | ≥80 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | Pa | |
| All respondents | 13902 | 100.0 | 561 | 100.0 | 5893 | 100.0 | 5971 | 100.0 | 1477 | 100.0 | <0.001 |
| Sexual activity | 3657 | 26.3 | 260 | 46.4 | 2112 | 35.8 | 1152 | 19.3 | 133 | 9.0 | |
| Self-stimulation only | 757 | 5.5 | 29 | 5.2 | 326 | 5.5 | 327 | 5.5 | 75 | 5.1 | |
| No sexual activity | 7356 | 52.9 | 206 | 36.7 | 2627 | 44.6 | 3514 | 58.9 | 1009 | 68.3 | |
| No response | 2132 | 15.3 | 66 | 11.8 | 828 | 14.1 | 978 | 16.4 | 260 | 17.6 | |
| Married or intimate partner | 7453 | 100.0 | 380 | 100.0 | 3718 | 100.0 | 2892 | 100.0 | 463 | 100.0 | <0.001 |
| Sexual activity | 3357 | 45.0 | 235 | 61.8 | 1943 | 52.3 | 1065 | 36.8 | 114 | 24.6 | |
| Self-stimulation only | 266 | 3.6 | 10 | 2.6 | 128 | 3.4 | 115 | 4.0 | 13 | 2.8 | |
| No sexual activity | 2638 | 35.4 | 95 | 25.0 | 1104 | 29.7 | 1200 | 41.5 | 239 | 51.6 | |
| No response | 1192 | 16.0 | 40 | 10.5 | 543 | 14.6 | 512 | 17.7 | 97 | 21.0 | |
| No spouse or intimate partner | 6448 | 100.0 | 180 | 100.0 | 2175 | 100.0 | 3079 | 100.0 | 1014 | 100.0 | <0.001 |
| Sexual activity | 300 | 4.7 | 25 | 13.9 | 169 | 7.8 | 87 | 2.8 | 19 | 1.9 | |
| Self-stimulation only | 491 | 7.6 | 19 | 10.6 | 198 | 9.1 | 212 | 6.9 | 62 | 6.1 | |
| No sexual activity | 4718 | 73.2 | 111 | 61.7 | 1523 | 70.0 | 2314 | 75.2 | 770 | 75.9 | |
| No response | 939 | 14.6 | 25 | 13.9 | 285 | 13.1 | 466 | 15.1 | 163 | 16.1 | |
P value from a logistic regression model with sexual activity (with partner or self-stimulation) as a function of linear trend over age categories (<60=1, 60–69=2, 70–79=3, etc.) among participants who answered the sexual activity question.
Characteristics of sexual activity among respondents who reported any sexual activity postintervention are presented in Table 7. For each of the seven characteristics, the majority (62%–88%) of EPT trial participants overall indicated no change, regardless of intervention arm, with over 75% of the EPT trial participants reporting their satisfaction with sexual activity was the same or greater since stopping study pills. However, there were statistically significant differences by intervention arm for each item. A decreased frequency of intercourse postintervention was more likely to be reported by women in the EPT group (20%) than in the placebo group (9%). Compared with the intervention period, women assigned to EPT were more likely than those assigned to placebo to report a decrease in desire (17% vs 6%), arousal (17% vs 7%), ability to climax (19% vs 7%), and satisfaction with sexual activity (17% vs 8%), as well as an increase in tightness of vagina (12% vs 3%) and discomfort with intercourse (15% vs 3%).
TABLE 7.
Characteristics of sexual activity postintervention among those who reported sexual activity, by trial participation and intervention arm
| Estrogen-Progestin Therapy (EPT) Trial | Estrogen Therapy (ET) Trial | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| EPT | Placebo | ET | Placebo | ||||||||
| n | % | n | % | Pa | n | % | n | % | Pa | ||
| Frequency of intercourseb | <0.001 | Frequency of intercoursec | 0.06 | ||||||||
| Greater | 47 | 3.6 | 20 | 1.6 | A lot of the time | 94 | 20.8 | 52 | 14.6 | ||
| Same or Not Applicable | 962 | 74.2 | 1112 | 86.7 | Some of the time | 302 | 66.8 | 262 | 73.8 | ||
| Less | 257 | 19.8 | 114 | 8.9 | Rarely or not at all | 42 | 9.3 | 35 | 9.9 | ||
| No response | 30 | 2.3 | 37 | 2.9 | No response | 14 | 3.1 | 6 | 1.7 | ||
| Discomfort with intercourseb | <0.001 | Discomfort with intercoursec | 0.26 | ||||||||
| Greater | 198 | 15.3 | 43 | 3.4 | A lot of the time | 32 | 7.1 | 23 | 6.5 | ||
| Same or Not Applicable | 974 | 75.2 | 1130 | 88.1 | Some of the time | 81 | 17.9 | 81 | 22.8 | ||
| Less | 48 | 3.7 | 30 | 2.3 | Rarely or not at all | 312 | 69.0 | 235 | 66.2 | ||
| No response | 76 | 5.9 | 80 | 6.2 | No response | 27 | 6.0 | 16 | 4.5 | ||
| Level of sexual desired | <0.001 | Sexual desiree | <0.001 | ||||||||
| Greater | 76 | 1.9 | 34 | 0.8 | A lot of the time | 134 | 7.5 | 72 | 4.0 | ||
| Same or Not Applicable | 2545 | 63.7 | 3151 | 75.2 | Some of the time | 702 | 39.5 | 620 | 34.3 | ||
| Less | 671 | 16.8 | 257 | 6.1 | Rarely or not at all | 788 | 44.3 | 926 | 51.3 | ||
| No response | 704 | 17.6 | 749 | 17.9 | No response | 154 | 8.7 | 187 | 10.4 | ||
| Feeling arousedd | <0.001 | Feeling arousede | <0.001 | ||||||||
| Greater | 76 | 1.9 | 30 | 0.7 | A lot of the time | 104 | 5.8 | 53 | 2.9 | ||
| Same or Not Applicable | 2490 | 62.3 | 3082 | 73.5 | Some of the time | 612 | 34.4 | 534 | 29.6 | ||
| Less | 695 | 17.4 | 301 | 7.2 | Rarely or not at all | 856 | 48.1 | 970 | 53.7 | ||
| No response | 735 | 18.4 | 778 | 18.6 | No response | 206 | 11.6 | 248 | 13.7 | ||
| Ability to climaxf | <0.001 | Ability to climaxg | 0.98 | ||||||||
| Greater | 55 | 3.3 | 26 | 1.6 | A lot of the time | 231 | 38.1 | 192 | 38.7 | ||
| Same or Not Applicable | 1223 | 73.9 | 1450 | 87.5 | Some of the time | 252 | 41.6 | 204 | 41.1 | ||
| Less | 312 | 18.9 | 112 | 6.8 | Rarely or not at all | 105 | 17.3 | 87 | 17.5 | ||
| No response | 64 | 3.9 | 70 | 4.2 | No response | 18 | 3.0 | 13 | 2.6 | ||
| Tightness of vaginaf | <0.001 | Tightness of vaginag | 0.12 | ||||||||
| Greater | 204 | 12.3 | 43 | 2.6 | A lot of the time | 59 | 9.7 | 65 | 13.1 | ||
| Same or Not Applicable | 1244 | 75.2 | 1456 | 87.8 | Some of the time | 124 | 20.5 | 109 | 22.0 | ||
| Less | 111 | 6.7 | 67 | 4.0 | Rarely or not at all | 391 | 64.5 | 295 | 59.5 | ||
| No response | 95 | 5.7 | 92 | 5.5 | No response | 32 | 5.3 | 27 | 5.4 | ||
| Satisfaction with sex activityf | <0.001 | Satisfaction with sex activityg | 0.52 | ||||||||
| Greater | 61 | 3.7 | 29 | 1.7 | A lot of the time | 280 | 46.2 | 223 | 45.0 | ||
| Same or Not Applicable | 1250 | 75.6 | 1443 | 87.0 | Some of the time | 246 | 40.6 | 192 | 38.7 | ||
| Less | 286 | 17.3 | 126 | 7.6 | Rarely or not at all | 70 | 11.6 | 68 | 13.7 | ||
| No response | 57 | 3.4 | 60 | 3.6 | No response | 10 | 1.7 | 13 | 2.6 | ||
P values for arm by survey are based on known survey responses only.
Frequencies among 2579 EPT trial participants who reported having intercourse since stopping study pills.
Frequencies among 807 ET trial participants who reported having intercourse in the three months prior to survey.
Frequencies among 8187 EPT trial participants who answered whether or not they had sexual activity since stopping study pills.
Frequencies among 3583 ET trial participants who answered whether or not they had sexual activity in the three months prior to survey.
Frequencies among 3312 EPT trial participants who reported sexual activity since stopping study pills.
Frequencies among 1102 ET trial participants who reported sexual activity in the three months prior to survey.
Unlike the EPT trial results, only two of the sexual function items in the ET trial yielded differences by intervention arm that were statistically significant. Both desire and arousal were reported to be rarely or not at all present more frequently among women formerly assigned to placebo than to ET (Table 7). In the ET trial a majority (>66%) of those reporting intercourse in the last three months indicated they had dyspareunia rarely or not at all, and 7% indicated they had dyspareunia a lot of the time. There was no statistically significant difference between those who had been randomized to ET and those randomized to placebo with regard to dyspareunia. ET trial respondents who reported discomfort with intercourse a lot of the time were asked if they thought the discomfort was due to dryness or tightness. Approximately 56% selected both dryness and tightness, 33% selected dryness only and 2% reported tightness only (data not shown). Uncertainty, reflected with a response of do not know, was given 5% of the time for dryness and 15% of the time for tightness.
Table 8 presents a cross-sectional view of sexual function in the ET trial by age groups. Although frequency of sexual desire, sexual arousal, ability to reach climax, and satisfaction with sexual activity decreased by age groups among sexually active participants, only satisfaction with sexual activity showed a statistically significant difference in the effect of ET over age groups. Overall, 85% of women indicated that they were satisfied with sexual activity a lot or some of the time. From 72% to 85% of women reported the ability to climax was experienced a lot or some of the time across all age groups with only a small decline. In a subgroup of sexually active women who indicated both low satisfaction and low interest in sex (n=72), 79% reported rare to no ability to climax, with the remainder of the sexually active cohort reporting rare to no ability to climax at a rate of 13%.
TABLE 8.
Sexual activity components postintervention among women in the Estrogen Therapy (ET) trial, by age postintervention and intervention arm
| <60 | 60–69 | 70–79 | ≥80 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | ET | Placebo | ET | Placebo | ET | Placebo | ET | Placebo | |||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | P Interactiona | |
| Sexual desire or interest | 0.09 | ||||||||||||||||
| A lot of the time | 7 | 14.3 | 6 | 12.0 | 71 | 10.0 | 48 | 7.3 | 48 | 6.1 | 17 | 2.1 | 8 | 3.5 | 1 | 0.4 | |
| Some of the time | 27 | 55.1 | 28 | 56.0 | 332 | 46.6 | 280 | 42.6 | 276 | 34.9 | 259 | 32.0 | 67 | 29.7 | 53 | 18.3 | |
| Rarely or not at all | 14 | 28.6 | 14 | 28.0 | 265 | 37.2 | 276 | 42.0 | 388 | 49.1 | 448 | 55.4 | 121 | 53.5 | 188 | 65.1 | |
| No response | 1 | 2.0 | 2 | 4.0 | 45 | 6.3 | 53 | 8.1 | 78 | 9.9 | 85 | 10.5 | 30 | 13.3 | 47 | 16.3 | |
| Feeling sexually aroused | 0.25 | ||||||||||||||||
| A lot of the time | 5 | 10.2 | 4 | 8.0 | 57 | 8.0 | 35 | 5.3 | 35 | 4.4 | 14 | 1.7 | 7 | 3.1 | 0 | 0.0 | |
| Some of the time | 30 | 61.2 | 23 | 46.0 | 294 | 41.2 | 256 | 39.0 | 234 | 29.6 | 212 | 26.2 | 54 | 23.9 | 43 | 14.9 | |
| Rarely or not at all | 12 | 24.5 | 20 | 40.0 | 308 | 43.2 | 299 | 45.5 | 416 | 52.7 | 468 | 57.9 | 120 | 53.1 | 183 | 63.3 | |
| No response | 2 | 4.1 | 3 | 6.0 | 54 | 7.6 | 67 | 10.2 | 105 | 13.3 | 115 | 14.2 | 45 | 19.9 | 63 | 21.8 | |
| Ability to reach climaxb | 0.06 | ||||||||||||||||
| A lot of the time | 12 | 44.4 | 15 | 48.4 | 120 | 37.9 | 105 | 43.8 | 82 | 37.3 | 61 | 32.8 | 17 | 40.5 | 11 | 28.2 | |
| Some of the time | 11 | 40.7 | 10 | 32.3 | 139 | 43.9 | 100 | 41.7 | 89 | 40.5 | 76 | 40.9 | 13 | 31.0 | 18 | 46.2 | |
| Rarely or not at all | 4 | 14.8 | 6 | 19.4 | 52 | 16.4 | 28 | 11.7 | 40 | 18.2 | 45 | 24.2 | 9 | 21.4 | 8 | 20.5 | |
| No response | 0 | 0.0 | 0 | 0.0 | 6 | 1.9 | 7 | 2.9 | 9 | 4.1 | 4 | 2.2 | 3 | 7.1 | 2 | 5.1 | |
| Satisfaction with sexual activityb | 0.02 | ||||||||||||||||
| A lot of the time | 15 | 55.6 | 15 | 48.4 | 143 | 45.1 | 122 | 50.8 | 103 | 46.8 | 74 | 39.8 | 19 | 45.2 | 12 | 30.8 | |
| Some of the time | 8 | 29.6 | 10 | 32.3 | 131 | 41.3 | 89 | 37.1 | 90 | 40.9 | 74 | 39.8 | 17 | 40.5 | 19 | 48.7 | |
| Rarely or not at all | 4 | 14.8 | 5 | 16.1 | 40 | 12.6 | 23 | 9.6 | 21 | 9.6 | 34 | 18.3 | 5 | 11.9 | 6 | 15.4 | |
| No response | 0 | 0.0 | 1 | 3.2 | 3 | 1.0 | 6 | 2.5 | 6 | 2.7 | 4 | 2.2 | 1 | 2.4 | 2 | 5.1 | |
Interaction P value for the interaction term from a linear model with the sexual variable of interest (1=Rarely or none at all; 2=Some of the time; 3=A lot of the time) as a function of ET intervention arm, linear age group (<60=1; 60–69=2; 70–79=3; 80–89=4), and their interaction.
Among participants who reported sexual activity.
Both EPT and ET trial respondents were given the opportunity to select symptoms they experienced after discontinuing their study pills from a long list of physical and psychological symptoms that had been administered at the original WHI enrollment baseline and other time points during the course of the study. Symptoms potentially related to GSM are listed in Table 9 along with the prevalence of dyspareunia some of the time or a lot of the time for the ET trial. Dyspareunia was the most common GSM symptom among 807 former ET and placebo users who reported having intercourse, 25% and 29%, respectively (p-value 0.21).
TABLE 9.
Prevalence of moderate to severe symptoms of genitourinary syndrome of menopause (GSM) postintervention, by trial participation and intervention arm
| Estrogen-Progestin Therapy (EPT) Trial | Estrogen Therapy (ET) Trial | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| EPT n=1654 |
Placebo n=1658 |
ET n=606 |
Placebo n=496 |
|||||||
| Participants reporting sexual activity | n | % | n | % | P | n | % | n | % | P |
| Any moderate/severe GSM symptoma | 321 | 19.4 | 170 | 10.3 | <0.001 | 152 | 25.1 | 136 | 27.4 | 0.38 |
| Vaginal irritation or itching | 75 | 4.5 | 49 | 3.0 | 0.05 | 20 | 3.3 | 17 | 3.4 | 0.66 |
| Vaginal dryness | 264 | 16.0 | 133 | 8.0 | <0.001 | 52 | 8.6 | 43 | 8.7 | 0.66 |
| Pain/burning with urination | 19 | 1.1 | 13 | 0.8 | 0.44 | 8 | 1.3 | 3 | 0.6 | 0.33 |
| Vaginal discharge | 28 | 1.7 | 11 | 0.7 | 0.02 | 5 | 0.8 | 4 | 0.8 | 0.66 |
| Vaginal spotting/bleeding | 16 | 1.0 | 8 | 0.5 | 0.20 | 2 | 0.3 | 0 | 0.0 | 0.29 |
| Dyspareunia (some or a lot)b | 113 | 25.0b | 104 | 29.3b | 0.21 | |||||
| EPT n=2342 |
Placebo n=2533 |
ET n=1172 |
Placebo n=1309 |
|||||||
| Participants reporting no sexual activity | N | % | N | % | P | N | % | n | % | P |
| Any moderate/severe GSM symptoma | 241 | 10.3 | 165 | 6.5 | <0.001 | 92 | 7.8 | 92 | 7.0 | 0.62 |
| Vaginal irritation or itching | 113 | 4.8 | 66 | 2.6 | <0.001 | 39 | 3.3 | 47 | 3.6 | 0.80 |
| Vaginal dryness | 144 | 6.1 | 99 | 3.9 | 0.002 | 49 | 4.2 | 50 | 3.8 | 0.76 |
| Pain/burning with urination | 32 | 1.4 | 34 | 1.3 | 0.99 | 16 | 1.4 | 15 | 1.1 | 0.75 |
| Vaginal discharge | 30 | 1.3 | 14 | 0.6 | 0.03 | 8 | 0.7 | 9 | 0.7 | 0.85 |
| Vaginal spotting/bleeding | 18 | 0.8 | 4 | 0.2 | 0.006 | 2 | 0.2 | 0 | 0.0 | 0.28 |
| EPT n=4599 |
Placebo n=4851 |
ET n=2193 |
Placebo n=2259 |
|||||||
| All participantsc | n | % | N | % | P | N | % | N | % | P |
| Any moderate/severe GSM symptoma | 639 | 13.9 | 382 | 7.9 | <0.001 | 279 | 12.7 | 264 | 11.7 | 0.57 |
| Vaginal irritation or itching | 218 | 4.7 | 132 | 2.7 | <0.001 | 71 | 3.2 | 79 | 3.5 | 0.89 |
| Vaginal dryness | 454 | 9.9 | 259 | 5.3 | <0.001 | 121 | 5.5 | 113 | 5.0 | 0.74 |
| Pain/burning with urination | 60 | 1.3 | 54 | 1.1 | 0.47 | 28 | 1.3 | 26 | 1.2 | 0.93 |
| Vaginal discharge | 65 | 1.4 | 29 | 0.6 | <0.001 | 17 | 0.8 | 15 | 0.7 | 0.91 |
| Vaginal spotting/bleeding | 45 | 1.0 | 14 | 0.3 | <0.001 | 8 | 0.4 | 4 | 0.2 | 0.48 |
| Dyspareunia (some or a lot)b | 113 | 25.0b | 104 | 29.3b | 0.21 | |||||
Includes dyspareunia for ET trial participants only.
Percentages calculated for ET participants who reported intercourse in the last 3 months (n=452 ET, 355 Placebo); rate could not be calculated for EPT participants, see Table 6.
Includes participants who did not indicate their sexual activity status.
EPT, estrogen-progestin therapy.
ET, estrogen therapy.
The overall prevalence of GSM, including dyspareunia, in the ET trial, was 25% to 27% among sexually active women, 7%–8% among women reporting no sexual activity, and 12%–13% among all women in the ET cohort. GSM prevalence that included dyspareunia could not be calculated for the EPT trial.
Excluding dyspareunia, the most commonly reported moderate/severe GSM symptom was vaginal dryness, which was reported by 11% of sexually active women, with a higher rate (16%) occurring among sexually active women who discontinued EPT (Table 9). Vaginal irritation or itching, discharge, spotting, and dysuria were each reported by fewer than 5% of sexually active respondents. Among women indicating no sexual activity, the report of symptoms potentially related to GSM was lower, with only 8% of women reporting at least one moderate/severe GSM symptom, most commonly vaginal dryness (5%). Apart from dyspareunia and vaginal dryness, rates of all other GSM symptoms were low and similar in both sexually active and inactive women.
The prevalence of vaginal dryness at the original WHI enrollment baseline reported by all sexually active women was 13%. At one year the rate of vaginal dryness was 12% among those assigned to placebo versus 8% among those assigned to HT. Postintervention, the rate was 14% among those formerly assigned to HT and 8% among those assigned to placebo (See Supplemental Digital Content Table 1 for data).
Hormone and nonhormone products were used postintervention to alleviate symptoms of menopause and GSM (See Supplemental Digital Content Table 2 for data). Sexually active women formerly assigned to EPT or ET were most likely to use vaginal lubricants (21.4%, 21.7%, respectively), which they used at a slightly higher rate than their placebo counterparts (16.4%, 19.0%, respectively) (p<0.001 for EPT trial). Similarly, sexually active women formerly assigned to EPT or ET were more likely to use HT (5.1%, 8.1%, respectively) than their placebo counterparts (1.4%, 1.0%, respectively) (p<0.001). Use of vaginal estrogens was very similar (2.0% to 2.8%) across all sexually active respondents.
Among sexually inactive women in the EPT and ET trials, 4.2% and 4.6%, respectively, used vaginal lubricants with no significant difference between former HT and placebo groups. Sexually inactive women formerly assigned to EPT or ET were more likely to use systemic HT (3.6%, 3.3%, respectively) than their placebo counterparts (0.6%, 0.8%, respectively), (p<0.001). Rates of vaginal estrogen use were similar among all sexually inactive women (1.0%–1.7%).
The majority of women using a vaginal lubricant or hormone therapy indicated it was useful (See Supplemental Digital Content Table 2 for data). Among all trial participants, use of herbal products for any symptom of menopause, including GSM, ranged from 1.7% to 3.5%, and use of dehydroepiandrosterone was <1.0%.
Women could select one or more reasons for using any form of HT postintervention. The most commonly selected reason was to deal with symptoms, reported by 49.6% of respondents. Type of symptom was not determined. Other reasons for using HT were advice from health care provider (43.1%), to feel better (36.5%), to prevent various diseases (21.5%), to look better (9.1%), and other (28.6%).
Discussion
This WHI postintervention analysis reveals mixed results from the EPT and ET trials regarding the prevalence of sexual activity after recent discontinuation of HT. In the EPT trial there was no statistically significant difference in prevalence of sexual activity between former EPT and placebo users. This finding is similar to findings from the intervention phase of the HT trials.14 In the ET trial, however, there was a small but statistically significant greater prevalence of sexual activity among women formerly randomized to ET compared with those formerly randomized to placebo. It was anticipated that a much higher percentage of women randomized to either EPT or ET compared with placebo would be sexually active at the end of the study and for some time postintervention. The most likely explanation for the modest effects of HT seen in this study is the more powerful impact of other factors such as lack of partner, partner’s inability to participate in sexual activity, co-morbidities, and the respondent’s reported lack of interest.
The statistically significant lower prevalence of sexual activity in the placebo group of the ET trial appears to have been driven by women who reported bilateral oophorectomy. Endogenous levels of estrone and testosterone are lower in women who have undergone bilateral oophorectomy compared with natural menopause.15 In the WHI Dietary Modification Trial, women who reported bilateral oophorectomy had a mean testosterone level 35% below that of women having at least one ovary.16 A report from the Study of Women’s Health Across the Nation indicated that testosterone, not estrogen, was positively associated with postmenopausal women’s sexual health.17 In the current study the presumed lower estrone and testosterone levels in ET trial women with no ovaries randomized to placebo may have decreased their ability to adapt sexually compared with women randomized to placebo with one or two ovaries. An independent role of hysterectomy on sexual function was not assessed.
Hysterectomy status was one of many differences between the two HT trial cohorts. There were demographic differences, as well as a higher prevalence of medical conditions among ET trial respondents (Table 1). The negative effect of medical conditions on sexual activity has been reported.18–20 Demographic factors associated with no sexual activity include low income level, less than high school education, higher body mass index, poor self-rated health, and no partner, all of which were found at higher rates among ET trial respondents. The different results between the two trials could be influenced by the timing of the analyses. Women in the ET trial were approximately 2 years older than the women in the EPT trial when they completed the postintervention survey. Additionally, the sexual activity response interval was different in the two questionnaires. Women in the ET trial were asked about sexual activity in the last three months while EPT trial participants were asked if they had been sexually active since stopping study pills, a timeframe of 8–12 months.
Differences between the two HT trials in the seven characteristics of sexual activity were most likely the result of the different response options in the two postintervention surveys. This report indicates an adverse effect on vaginal and sexual function symptoms in approximately 15% to 20% of women discontinuing EPT. Conversely, 15%–20% were deriving sexual function benefit while taking EPT. Exacerbation of sexual function symptoms upon discontinuation of EPT would be expected among former ET users as well but was not captured. The duration of the recurrent symptoms was not determined. In a report on the recurrence of menopausal symptoms after discontinuation of HT in postmenopausal women under age 60, symptoms were reported to decrease over the 3 year follow-up.8
Symptoms of GSM were reported less frequently in this cohort (12% overall; 25% among sexually active women for any moderate to severe symptom in the ET trial) relative to other reports in the literature, some of which are higher (43%–57%).2,21–24 A possible explanation for the lower prevalence in the current report is that we focused on moderate to severe symptoms, the usual indication for treatment of menopausal symptoms. The higher symptom prevalence rates in the literature may have included mild symptoms which, if included in the current study, would bring the prevalence of any GSM symptom to 36% (See Supplemental Digital Content Table 3 for data).
A caveat for this and all reports on the prevalence of GSM or symptomatic vulvovaginal atrophy is that investigators do not always use the same list of symptoms. The definition of GSM used in this analysis included vaginal dryness, irritation, itching, discharge, spotting and bleeding, and dyspareunia, as well as pain and burning with urination. The survey combined vaginal irritation and itching into one question. Because some of these symptoms may be the result of other conditions such as candidiasis, contact dermatitis, lichen sclerosus, vestibulodynia/vulvodynia, and urinary tract infection, the prevalence of GSM in this population may have been overestimated. This report did not include all the lower urinary tract symptoms now included in GSM.4
Another explanation for the lower prevalence of vulvovaginal symptoms in this report is that this cohort was recruited for a general women’s health study as opposed to a women’s sexual health study. The context of a survey and its broad or narrow focus may affect results. The WHI questionnaires covered many aspects of life and health. The cohort was healthier than the general population because women with cancer or short life expectancy were excluded. Women with estrogen-related cancers would be likely to have higher rates of GSM as a result of radiation and/or estrogen deprivation therapy.
The percentage of women reporting vulvovaginal symptoms decreased by age decade over 60 years, a finding consistent with a report on 98,705 women in the WHI Observational Study and Clinical Trials.25 This decline may parallel the decrease in sexual activity and/or the decrease in the distress associated with various components of sexual activity with age.20,26,27
Despite a statistically significant difference in the decline in satisfaction with sexual activity between treatment arms by age groups, the ET trial demonstrates that both satisfaction and orgasmic capacity were present at higher levels across age groups than were sexual desire and sexual arousal. Although the non-response rate was as high as 20% for some questions, only 3% of women did not answer the orgasm and sexual satisfaction questions. The very high rate of rare-to-no orgasm among women with combined low satisfaction and low desire is clinically important, but causality within this triad of symptoms was not ascertained from the current data set.
Women’s reported reasons for no sexual activity harmonize with findings in other studies, with lack of partner being the most widely cited reason. However, assuming the “woman not able” reason would be selected by women unable to have intercourse because of severe symptoms of GSM, the 2.2% of women selecting this response suggests a very low percentage of women are in the severe GSM category. In addition to GSM, the not able category would be expected to include any physical, mental, or emotional limitations precluding intercourse. The reasons for no sexual activity had similar ranking order across active drug and placebo respondents in both HT trials.
There are several limitations to this report. The respondents represented survivors who were still taking study pills before the trial was stopped, regardless of adherence level. This may accentuate the effectiveness of the therapy as well as the withdrawal effects compared with an intent-to-treat analysis. However, results indicate what women can expect if they continue HT for an extended period of time. The surveys did not use a previously-validated sexual function questionnaire nor did they have a specific measure of symptom distress or bother other than the categories of mild, moderate and severe. Other limitations include the lack of a physical examination to eliminate other causes of vulvovaginal symptoms, high non-response rate to some sexual function questions, and lack of a relationship satisfaction question. Because the response options for the characteristics of sexual function were not identical in the two HT trial postintervention surveys, EPT and ET trial participants cannot be compared item for item on those seven factors. Different sexual activity and symptom outcomes between the two trials should not be attributed to potentially different effects of EPT or ET, because the original WHI enrollment baseline characteristics of the participants in the two HT trials were dissimilar.
The percentage of women in this report choosing to use HT products to treat symptoms postintervention was very low. Participants had recently been instructed to discontinue their study pills when the benefits of HT use for prevention of chronic disease did not appear to outweigh the risks. HT usage rates cited herein may underestimate current use of HT therapy, especially in regard to low-dose vaginal estrogen therapy for vaginal dryness and dyspareunia.8,28 Vaginal estrogen products may be more effective than systemic hormones for these symptoms.29
This report has several strengths including the large size of the cohort, the duration of HT use and follow-up, and the extensive characterization of participants with regard to other health issues and demographic information. These participants were diverse and recruited for a general women’s health study as opposed to a sexual function study. As such, the prevalence of GSM and sexual function symptoms reported here may more accurately reflect the prevalence in the general, relatively healthy, female population over age 55. The different format of the sexual function questions in the two trials provided complementary perspectives on the effect of HT discontinuation on GSM symptoms and sexual activity.
The similar prevalence rates of sexual activity between former EPT and placebo users suggest there is adaptation to lower estrogen levels with regard to sexual function in many women, particularly those with intact ovaries. Further research on the combined effects of endogenous postmenopausal estrogen and testosterone levels, the vaginal microbiome, and sexual activity patterns would add to the understanding of postmenopausal sexual activity.
Conclusions
There was no difference in the prevalence of sexual activity between former oral EPT and placebo users. In the ET trial, the women discontinuing oral ET reported a slightly higher prevalence of sexual activity compared with those who discontinued placebo. The statistical significance appeared to be driven by the lower rate of sexual activity among placebo users who reported bilateral oophorectomy.
More women who discontinued EPT compared with those who discontinued placebo reported symptoms of undetermined duration associated with GSM as well as a decrease in desire, arousal, ability to climax and satisfaction with sexual activity. Women who discontinued ET were less likely to report rare desire or arousal compared with those assigned to placebo. The majority of women in both HT trials maintained orgasmic function and reported sexual satisfaction postintervention. These findings support the need for further research to better explain how the majority of women adapt to sexual function in low estrogen states and why only some sexually active women are significantly affected by symptoms of GSM.
Supplementary Material
Acknowledgments
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, and HHSN268201600004C. Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, and Nancy Geller. Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg Investigators and Academic Centers: (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (MedStar Health Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Iowa, Iowa City/Davenport, IA) Jennifer Robinson; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker; (University of Nevada, Reno, NV) Robert Brunner; (University of Minnesota, Minneapolis, MN) Karen L. Margolis Women’s Health Initiative Memory Study: (Wake Forest University School of Medicine, Winston-Salem, NC) Mark Espeland. We thank the participants for their dedication to this study.
Funding: The Women’s Health Initiative program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services.
Footnotes
Conflicts of interest/financial disclosures: None.
Reprints: Not available.
References
- 1.Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause. 2013;20:888–902. doi: 10.1097/GME.0b013e3182a122c2. [DOI] [PubMed] [Google Scholar]
- 2.Santoro N, Komi J. Prevalence and impact of vaginal symptoms among postmenopausal women. J Sex Med. 2009;6:2133–2142. doi: 10.1111/j.1743-6109.2009.01335.x. [DOI] [PubMed] [Google Scholar]
- 3.The role of local vaginal estrogen for treatment of vaginal atrophy in postmenopausal women: 2007 position statement of The North American Menopause Society. Menopause. 2007;14:357–369. doi: 10.1097/gme.0b013e31805170eb. [DOI] [PubMed] [Google Scholar]
- 4.Portman DJ, Gass ML, Vulvovaginal Atrophy Terminology Consensus Conference Panel Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and The North American Menopause Society. Menopause. 2014;21:1063–1068. doi: 10.1097/GME.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 5.Ockene JK, Barad DH, Cochrane BB, et al. Symptom experience after discontinuing use of estrogen plus progestin. JAMA. 2005;294:183–193. doi: 10.1001/jama.294.2.183. [DOI] [PubMed] [Google Scholar]
- 6.Rolnick SJ, Kopher RA, DeFor TA, Kelley ME. Hormone use and patient concerns after the findings of the Women’s Health Initiative. Menopause. 2005;12:399–404. doi: 10.1097/01.GME.0000148644.55486.36. [DOI] [PubMed] [Google Scholar]
- 7.Brunner RL, Aragaki A, Barnabei V, et al. Menopausal symptom experience before and after stopping estrogen therapy in the Women’s Health Initiative randomized, placebo-controlled trial. Menopause. 2010;17:946–954. doi: 10.1097/gme.0b013e3181d76953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perrone G, Capri O, Galoppi P, et al. Menopausal symptoms after the discontinuation of long-term hormone replacement therapy in women under 60: a 3-year follow-up. Gynecol Obstet Invest. 2013;76:38–43. doi: 10.1159/000351104. [DOI] [PubMed] [Google Scholar]
- 9.Newton KM, Reed SD, Nekhyludov L, et al. Factors associated with successful discontinuation of hormone therapy. J Womens Health. 2014;23:382–388. doi: 10.1089/jwh.2012.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Women’s Health Initiative Study Group. Design of the Women’s Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 11.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 12.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 13.Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310:1353–1368. doi: 10.1001/jama.2013.278040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gass ML, Cochrane BB, Larson JC, et al. Patterns and predictors of sexual activity among women in the Hormone Therapy trials of the Women’s Health Initiative. Menopause. 2011;18:1160–1171. doi: 10.1097/gme.0b013e3182227ebd. [DOI] [PubMed] [Google Scholar]
- 15.Fogle RH, Stanczyk FZ, Zhang X, Paulson RJ. Ovarian androgen production in postmenopausal women. J Clin Endocrinol Metab. 2007;92:3040–3043. doi: 10.1210/jc.2007-0581. [DOI] [PubMed] [Google Scholar]
- 16.McTiernan A, Wu L, Barnabei VM, et al. Relation of demographic factors, menstrual history, reproduction and medication use to sex hormone levels in postmenopausal women. Breast Cancer Res Treat. 2008;108:217–231. doi: 10.1007/s10549-007-9588-6. [DOI] [PubMed] [Google Scholar]
- 17.Randolph JF, Jr, Zheng H, Avis NE, Greendale GA, Harlow SD. Masturbation frequency and sexual function domains are associated with serum reproductive hormone levels across the menopausal transition. J Clin Endocrinol Metab. 2015;100:258–266. doi: 10.1210/jc.2014-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Appa AA, Creasman J, Brown JS, et al. The impact of multimorbidity on sexual function in middle-aged and older women: beyond the single disease perspective. J Sex Med. 2014;11:2744–2755. doi: 10.1111/jsm.12665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laumann EO, Das A, Waite LJ. Sexual dysfunction among older adults: prevalence and risk factors from a nationally representative U.S. probability sample of men and women 57–85 years of age. J Sex Med. 2008;5:2300–2311. doi: 10.1111/j.1743-6109.2008.00974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shifren JL, Monz BU, Russo PA, Segreti A, Johannes CB. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112:970–978. doi: 10.1097/AOG.0b013e3181898cdb. [DOI] [PubMed] [Google Scholar]
- 21.Erekson EA, Li FY, Martin DK, Fried TR. Vulvovaginal symptoms prevalence in postmenopausal women and relationship to other menopausal symptoms and pelvic floor disorders. Menopause. 2016;23:368–375. doi: 10.1097/GME.0000000000000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nappi RE, Kokot-Kierepa M. Vaginal Health: Insights, Views & Attitudes (VIVA) - results from an international survey. Climacteric. 2012;15:36–44. doi: 10.3109/13697137.2011.647840. [DOI] [PubMed] [Google Scholar]
- 23.Levine KB, Williams RE, Hartmann KE. Vulvovaginal atrophy is strongly associated with female sexual dysfunction among sexually active postmenopausal women. Menopause. 2008;15:661–666. doi: 10.1097/gme.0b013e31815a5168. [DOI] [PubMed] [Google Scholar]
- 24.Stenberg A, Heimer G, Ulmsten U, Cnattingius S. Prevalence of genitourinary and other climacteric symptoms in 61-year-old women. Maturitas. 1996;24:31–36. doi: 10.1016/0378-5122(95)00996-5. [DOI] [PubMed] [Google Scholar]
- 25.Pastore LM, Carter RA, Hulka BS, Wells E. Self-reported urogenital symptoms in postmenopausal women: Women’s Health Initiative. Maturitas. 2004;49:292–303. doi: 10.1016/j.maturitas.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 26.Trompeter SE, Bettencourt R, Barrett-Connor E. Sexual activity and satisfaction in healthy community-dwelling older women. Am J Med. 2012;125:37–43. doi: 10.1016/j.amjmed.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayes RD, Dennerstein L, Bennett CM, Koochaki PE, Leiblum SR, Graziottin A. Relationship between hypoactive sexual desire disorder and aging. Fertil Steril. 2007;87:107–112. doi: 10.1016/j.fertnstert.2006.05.071. [DOI] [PubMed] [Google Scholar]
- 28.Tsai SA, Stefanick ML, Stafford RS. Trends in menopausal hormone therapy use of US office-based physicians, 2000–2009. Menopause. 2011;18:385–392. doi: 10.1097/gme.0b013e3181f43404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Long CY, Liu CM, Hsu SC, Wu CH, Wang CL, Tsai EM. A randomized comparative study of the effects of oral and topical estrogen therapy on the vaginal vascularization and sexual function in hysterectomized postmenopausal women. Menopause. 2006;13:737–743. doi: 10.1097/01.gme.0000227401.98933.0b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




