Abstract
Now that microplastics have been detected in lakes, rivers, and estuaries all over the globe, evaluating their effects on biota has become an urgent research priority. This is the first study that aims at determining the effect thresholds for a battery of six freshwater benthic macroinvertebrates with different species traits, using a wide range of microplastic concentrations. Standardized 28 days single species bioassays were performed under environmentally relevant exposure conditions using polystyrene microplastics (20–500 μm) mixed with sediment at concentrations ranging from 0 to 40% sediment dry weight (dw). Microplastics caused no effects on the survival of Gammarus pulex, Hyalella azteca, Asellus aquaticus, Sphaerium corneum, and Tubifex spp. and no effects were found on the reproduction of Lumbriculus variegatus. No significant differences in growth were found for H. azteca, A. aquaticus, S. corneum, L. variegatus, and Tubifex spp. However, G. pulex showed a significant reduction in growth (EC10 = 1.07% sediment dw) and microplastic uptake was proportional with microplastic concentrations in sediment. These results indicate that although the risks of environmentally realistic concentrations of microplastics may be low, they still may affect the biodiversity and the functioning of aquatic communities which after all also depend on the sensitive species.
Introduction
Microplastics, defined as plastic particles with a size <5 mm,1 have been detected in both terrestial and aquatic ecosystems.2 While their abundance and distribution in the marine environment have been found to be of great importance and have been covered already for a decade, their presence in terrestial and freshwater ecosystems is only recognized more recently.2−4 Nevertheless, a wide range of microplastics has been identified at different concentrations in water and sediment samples from lakes, rivers, and estuaries all over the globe.2−4
Key factors influencing the fate and transport of microplastics in freshwater systems are the type of aquatic system, as well as the climate conditions and plastic sources in the area.4−6 Moreover, microplastic properties such as size, density or shape, have a direct effect on the processes of biofouling and aggregation, affecting the sedimentation and resuspension of particles and, thus, the abundance of microplastic in the water column and sediments.4−6 In fact, particle size has been found to strongly affect the presence of microplastic hotspots along river sediments, indicating that sediments can act as a sink for microplastics.5 Recent data shows that the Rhine river contains the highest microplastic concentrations detected in all freshwater bodies studied. Concentrations up to 4000 particles kg–1 or 1 g kg–1 (dw) were recorded in the German Rhine river shore sediments, with the smallest microplastic fraction (63–630 μm) being the most abundant in numbers.7 In the Dutch area, up to 4900 particles kg–1 (dw) were accounted in the suspended particulate matter, in which 30% of the particles had a size between 10 and 300 μm and 70% were bigger.8
Understanding the interaction between microplastics and biota in freshwater systems has been identified as a high priority research need4 and there is a general agreement on the idea that an effect assessment should be performed to evaluate the risk of exposure to microplastics.9 This is especially important in the case of freshwater benthic organisms, that seem to have a higher risk of exposure due to the sinking of microplastics onto sediments.5 Previous studies have indeed demonstrated that microplastics are taken up from sediments by freshwater species10−15 and that the capacity of freshwater invertebrates to ingest microplastics depends on their feeding type.15 Moreover, this uptake was related to a decrease in the growth of Gammarus fossarum exposed to poly(methyl methacrylate) (PMMA) and polyhydroxybutyrate (PHB) microplastics.12 Also, a reduction in the growth and reproduction of Hyalella azteca was found after the exposure to polyethylene (PE) microplastics.11 However, no effects were reported on the survival, molting, metabolism and feeding activity of Gammarus pulex after the uptake of polyethylene terephtalate (PET) microplastics.14 Microplastic uptake did not cause any effects on the marine isopod Idotea emarginata(16) but did cause weight loss and a reduced feeding activity for the marine lugworm Arenicola marina.17−19 No or limited effects have also been found for other marine invertebrates.20,21 This suggests that benthic macroinvertebrates are affected by the presence of microplastics but also that the susceptibility could be species specific.
Current studies have mainly focused on the ingestion of microplastics;3 however, the quality, reliability, and usability of the few ecological effect data published have been put into question.22,23 The use of nonstandardized laboratory bioassays and unrealistic exposure scenarios hinders the understanding of the risks associated with microplastics.9,22,23 Furthermore, it remains unclear if adverse effects are caused by a physical impact of the particles themselves, by chemical toxicity or by a combination of both.2 Moreover, an effect assessment for microplastics should aim at detecting the effect thresholds for traditional end points in ecotoxicology (i.e., LC50 or EC50),22 and for this, sufficient doses and replication are needed in order to fit dose–response models, which are commonly used in chemical risk assessment.9
In the present study we aim at determining the effect thresholds for a battery of freshwater benthic macroinvertebrates with different species-specific traits, using a wide range of microplastic concentrations. Standardized 28 days single species bioassays were performed under environmentally relevant exposure conditions using polystyrene (PS) microplastics (20–500 μm) mixed with sediment at concentrations ranging from 0 to 40% plastic in sediment dw. We did not aim to assess chemical effects as this has been dealt with in many earlier studies, for example, refs (18, 19, and 24), and because it has been argued recently that chemical risks of microplastics should be separated from risks associated with physical effects.9 We are not aware of earlier studies systematically assessing microplastic effect thresholds for a range of organisms. Effects of PS microplastics on mortality and growth were assessed for six benthic freshwater macroinvertebrates: Gammarus pulex, Hyalella azteca, Asellus aquaticus, Sphaerium corneum, Lumbriculus variegatus, and Tubifex spp. Effects of PS microplastics on feeding activity was also assessed as feeding rate for G. pulex, H. azteca, and A. aquaticus and as egestion rate for Tubifex spp. and L. variegatus. Moreover, for G. pulex and H. azteca, the presence of PS microplastics in the faecal pellets and in their bodies after 24 h defeacation was asessed in order to investigate if the differences in the effects caused by the exposure to microplastics on both species were related with their ingestion and egestion mechanisms. For all end points, environmentally relevant exposure conditions were simulated by using natural sediments and by including the highest concentration found in a freshwater sediment. Polystyrene, ground to a wide and environmentally relevant range of sizes and shapes, was considered as a fair approximation to assess the physical effects of “environmental microplastics”.7 After all, polystyrene density matches that of the average environmental microplastic5,25 (see calculation in Supporting Information (SI) Table S1) and polymer density has limited impact on physical effects. Any potential additives present were removed from the microplastics to eliminate any ambiguity concerning what caused the effect of the particles.
Materials and Methods
Microplastics
Irregular polystyrene fragments were provided in a powdered form by Axalta Coating Systems GMBH (Cologne, Germany). Particle size distribution (PSD), measured with a Mastersizer 3000 (Malvern Instruments), revealed an unimodal distribution spanning from 20 to 500 μm, with a modus centered at 229 μm in volume and 36 μm in number (SI Figure S1). To remove additives present, if any, the microplastics were washed with methanol three times, shaken, filtered with a 20 μm metal sieve and dried for at least 2 days in a fume hood at room temperature. Polymer identity and purity were confirmed with FTIR spectrometry (Nicolet iN10, ThermoFisher) and particle shape was confirmed with an Olympus SZX10 stereomicroscope (Figure S2, SI).
Test Organisms
Species selected were the amphipods Gammarus pulex (Linnaeus, 1758) and Hyalella azteca (Saussure, 1858), the isopod Asellus aquaticus (Linnaeus, 1758), the bivalve Sphaerium corneum (Linnaeus, 1758), and the worms Lumbriculus variegatus (Müller, 1774) and Tubifex spp. (Lamark, 1816). These species are common members of freshwater communities, are often used in laboratory experiments and differ in their living and feeding strategies, as well as in their sensitivity to environmental pollutants.26−28G. pulex, H. azteca, and A. aquaticus are regarded as being mainly shredders, whereas S. corneum is classified as a facultative suspension feeder. S. corneum is an epibenthic species that lives and feeds on the sediment, whereas G. pulex and H. azteca are also active swimmers. L. variegatus and Tubifex spp. are both endobenthic deposit feeders, with L. variegatus regarded as a bulk feeder while Tubifex spp. exhibits selectivity in its feeding behavior.29
Following previously published procedures,27,30G. pulex, A. aquaticus, and S. corneum were collected from an unpolluted brook (Heelsum, The Netherlands), a ditch (Heteren, The Netherlands) and a pond (Renkum, The Netherlands), respectively. H. azteca and L. variegatus were obtained from Wageningen Environmental Research (Wageningen, The Netherlands) and Tubifex spp. were obtained from a local pet shop. Prior to the experiments, organisms were acclimatized for 2 weeks in aerated buckets with copper-free Dutch Standard Water (DSW) inside a water bath at 16 ± 1 °C while maintaining a 12:12 light:dark cycle. During the acclimatization, G. pulex, A. aquaticus, and H. azteca were fed with dry poplar leaves that were collected in the field and S. corneum, L. variegatus, and Tubifex spp. were fed with TetraMin fish food pellets.
Sediments
Freshwater sediments were collected from a noncontaminated ditch in Veenkampen (Wageningen, The Netherlands) using a standard dip net. Background concentrations of ∑PAH and ∑PCBs were factors of >8 and >70 below toxicity thresholds,27 whereas heavy metals were below negligible risk levels according to Dutch sediment quality criteria (SI Table S2). Sediments were passed over a 2 mm sieve, homogenized and placed in a freezer to kill any organisms present and to preserve the total organic matter (TOM) content. Prior to the experiments, sediments were thawed and homogenized again. Four representative subsamples were taken to determine the % TOM through loss on ignition (3 h, 550 °C), which was 31.6% ± 3.5 (n = 4).
Experimental Design
Bioassay experimental units consisted of 750 mL glass beakers filled with 211 g of wet sediment and 300 mL of copper-free DSW. Polystyene microplastics were added to the sediment to obtain eight final uniform concentrations of 0, 0.1, 1, 5, 10, 20, 30 and 40% plastic weight in the total sediment mixture. Concentrations ranged from environmentally relevant (0–1% plastic weight in sediment dw) to very high concentrations, to evaluate dose–response relations and to maximize the chance of accurately detecting the effect threshold.9 Four replicates of each concentration were made, except for H. Azteca, for which only three replicates were made and for which the concentration of 5% was excluded. Beakers with the suspension of sediment and PS were manually shaken to overcome the energy barrier to settling due to the surface tension (if any), after which particles settled within 48 h. Two weeks prior to the start of the experiment, beakers were placed in a water bath at a constant temperature of 16 ± 1 °C and accommodated with aeration.
At the start of the experiment, 11 randomly selected individuals were placed in their corresponding beakers. The size range of G. pulex, A. aquaticus, and S. corneum was between 4 and 7 mm and for H. azteca between 1 and 3 mm. Active adult worms with an average wet weight of 3.2 and 12.4 mg per worm were selected for Tubifex spp. and L. variegatus, respectively. The starting length and weight of 44 randomly selected individuals from the initial population were assessed. During the experiments, two poplar leaves discs with a diameter of 3 cm were supplied to the beakers of G. pulex, A. aquaticus, and H. azteca at day 0 and 14. Poplar leaves discs were previously conditioned with DSW for 3 days. For S. corneum, a TetraMin suspension of 0.5 mg per individual per day was added every 3 days. No additional food was needed for L. variegatus and Tubifex spp. due to the high organic matter content of the sediment. Dissolved oxygen (DO), pH, temperature, conductivity, and NH3 were measured in at least one replica of each concentration at day 0, 3, 7, 14, 17, 21, 24, and 28. To keep water levels constant, DSW was added weekly until the end of the experiment. Water quality variables remained constant in all beakers along the experiment (Table S3, SI), except for the treatments with Tubifex spp. and L. variegatus where pH approached values below the recommended limits (6–9) at day 14 and 24, respectively.31 This was solved by replacing 100 mL from the surface water layer in the bioassay by fresh DSW. On average, the measured temperature was 16 ± 0.3 °C, pH was 7.3 ± 0.5, oxygen concentration was 8.9 ± 0.2 mg/L and conductivity was 477 ± 45 μS/cm. Un-ionised levels of ammonia decreased along the experiment for all species, reaching an average of 0.002 ± 0.001 mg NH3/l at the end of the experiment. All un-ionized ammonia levels were always below the LC50 values available for these species.26,32−34
Mortality and Growth
After 28 days, each system was sieved and the surviving organisms were collected, counted and transferred to clean DSW water to depurate their gut for 24 h. Thereafter, G. pulex, A. aquaticus, and S. corneum were preserved in ethanol 80% and their length was measured: shell length of S. corneum, body lengh of A. aquatics, and head capsule (HD in mm) of G. pulex from which total length (TL) was calculated as TL = −2.07 + 9.82 HD.35 Growth was determined as the difference in mean length (in mm) of the animals in each replicate at the end minus the mean length from 44 animals at the start of the experiment. For H. azteca, L. variegatus, and Tubifex spp., growth was measured as a difference in dry weight (in mg) of the population at the start and at the end of the experiment.
Feeding Activity of G. pulex, H. azteca, A. aquaticus, L. variegatus, and Tubifex spp
Feeding rates: The feeding rate (mg dw leaf/organism/d) of G. pulex, H. azteca, and A. aquaticus was calculated from the loss of the added poplar leaves using the following equation:36
| 1 |
where L1 is the initial and L2 the final dry weight of the Populus sp. disc (mg), Li1 and Li2 are the numbers of living organisms at the start and at the end of the experiment (Li1 = 11 individuals), Cl is the leaching-decomposition correction factor, calculated by dividing the initial dry weight by the final dry weight of the leaves in the control sample; and t is the incubation time (days).
Egestion rates: The egestion rate of L. variegatus and Tubifex spp. was assessed in a separate 15 day bioassay experiment following Leppanen and Kukkonen (1998)37 assuming that the weight of the faecal pellets represents the feeding rate of worms.38 Another batch of experimental systems was made following the same procedure as for the mortality and growth tests but now only three concentrations were prepared (0, 0.1, and 40%) in quadruplicate. After 2 weeks of acclimatization (with aeration, in a water bath at 16 ± 0.3 °C, with 12:12 light:dark cycle) to promote settling of particles, five active worms were added to these bioassays. When all organisms appeared to be buried in the sediment, a sand layer of 2 mm thickness and with a particle size between 0.5–1 mm was added. The egested pellets of the organisms were collected with a pipet at day 2, 5, 7, 12, and 15 for L. variegatus and at day 1, 5, 7, 12, and 15 for Tubifex spp. The collected faecal pellets were kept at 5 °C until they were filtered with GF/F 0.7 μm glass filters and dried at 60 °C for 48h and weighed.23 At the end of the experiment, worms were gathered and placed in clean DSW water to clear their gut content for 24 h. Finally, wet weight (ww) and dry weight (dw) (heating at 60 °C for 48 h) were determined per replicate. The egestion rate was calculated as the amount of faeces produced per worm per hour (mg dw per worm per h). No mortality occurred during the 15 day experiment.
Ingestion, Retention and Egestion of Polystyrene Microplastic by G. pulex and H. azteca
The presence of microplastics in bodies of G. pulex and H. azteca and in their faecal pellets was checked at the end of the 28 day bioassays. Samples were digested with 30% H2O2 and incubated at 60 °C in a water bath for 48 h39 following a protocol modified from Claessens et al. (2013). Afterward, samples were filtered through 25 mm Anodisc inorganic filter membranes of 0.2 μm pore size, which were dried in an oven at 50 °C for at least 48 h and analyzed with a micro-Fourier transform infrared spectrophotometer (μ-FTIR; Nicolet iN10, ThermoFisher) with a single MCT- detector and ultrafast stage. Following Mintenig et al. (2016),40 four predetermined and equally sized chemical maps covering one-third of the total filter area were made using an aperture size of 50 × 50 μm and mapping stage step sizes of 20 μm. A correlation map between the analyzed area and the spectra from the original PS microplastic sample was made with the OMNIC PICTA Software to determine identity, number and size of the ingested and excreted particles. The number of microplastics in the body of the organisms (retained microplastics) and the number of particles found in their faecal pellets (egested microplastics) were calculated for each treatment replicate. Organisms and faecal pellets from the 1% treatment, as well as one replica of the 30% treatment, were used to optimize the extraction of microplastics and, therefore, their data were omitted from further analysis. Organisms from four control beakers (exposed to sediment without PS microplastics) were used as blanks and were also checked for the presence of any microplastics, which were considered as contamination. The numbers of particles found in controls for the retained and egested particles were subtracted from the numbers of particles found in each replica of each PS treatment, and were 0.10 and 0.6 particles per organism, respectively.
Ingestion and egestion data were expressed on a microplastic particle number as well as on a weight basis, per unit of weight of organism and sediment. Microplastic number concentrations were directly taken from the FTIR mapping data. Weight based data require a number to weight conversion, for which we used an approximated volume of the particles, and the default density of PS (1.05 g/cm3).41 The approximate volumes (length × width × depth) of the PS microplastic fragments were calculated as follows. First it was assumed that the particles would prefer a flat position on the filter, such that their length and width dimensions directly measurable from the 2D map top view, each are larger than the third (depth) dimension, which is not observable from the 2D maps. This unknown third dimension was assumed to be the smallest and was thus approximated as half of the second dimension. We emphasize that this method is not accurate for individual particles, but becomes robust when it concerns larger numbers of our irregularly shaped particles where the distribution of the third (depth) dimension can be assumed to be symmetrical. The dry weight (DW in mg) of the organisms of G. pulex was estimated based on their lengh (L in mm) as DW = 0.00321 × L2.8309.42 The number of particles per gram of sediment was calculated from the mass of PS per dose, PS density and the measured particle volume distribution (SI Figure S2).
Data Analysis
Survival data as quantal data were analyzed using generalized linear model (GLMs) with a binomial distribution and probit model.43 One-way analysis of variance (ANOVA) and regression analysis were used to evaluate the effects of increasing microplastic concentrations in sediment on the growth, feeding rate and microplastic retention and egestion. Normality of the residuals and homogeneity of variances were tested with a Shapiro-Wilk Normality test and Levene’s test, respectively. Repeated measures (RM) ANOVA was used to determine the effects on the egestion rate over time. All statistical data analyses were conducted using SPSS 23 (IBM Corp., NY). When a significant effect on an end point was found, a four parameter log–logistic dose–response model was fitted;44
| 2 |
with f(x,b,c,d,e) is the bioassay response variable, x is the microplastic concentration, e is the median effect dose (EC50) and b,c,d are fitting parameters. In case parameter c was zero, eq 2 was reduced to a three-parameter model.44 The s.d. of the EC50 was calculated as the 95% confidence interval (CI95) divided by 1.96, where CI95 was calculated according to Draper and Smith (1981).30,45 The EC10 was calculated by solving the parametrized response model for a 10% effect dose.
Results and Discussion
Mortality
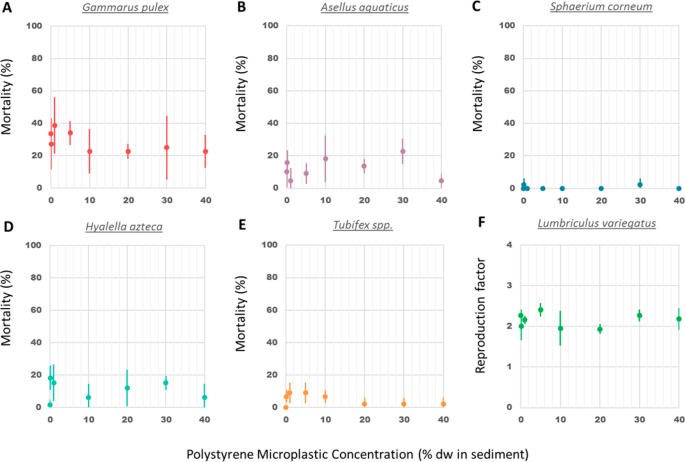
Mortality of L. variegatus could not be determined due to their reproduction by fragmentation during the experiment. As the average number of surviving worms per replicate in controls was increased by a factor of at least 1.8, the reproduction factor could be calculated as the number of worms at the end of the experiment divided by the number of worms at the beginning of the experiment.31 Survival for the other species was higher than 80% in controls, except for G. pulex, for which the average survival was 66%.
Chronic exposure to PS microplastic concentrations up to 40% in sediment dw caused no significant mortality in G. pulex, A. aquaticus, S. corneum, H. azteca, and Tubifex spp. (Figure 1A–E), and no significant effects were found on the reproduction of L. variegatus (Figure 1F). Same lack of effects on mortality has been reported in earlier studies with benthic macroinvertebrates exposed to microplastics. For instance, survival of the freshwater amphipods G. pulex and H. azteca were not affected by the exposure to PET and PE microplastics, respectively.11,14 Furthermore, no mortality was reported for the marine isopod Idotea emarginata exposed to PE, PS, and PA (polyamide) microplastics and the marine lugworm A. marina exposed to PE microplastics.19
Figure 1.
Mean mortality (±SD) for G. pulex (A), A. aquaticus (B), S. corneum (C), H. azteca (D), Tubifex spp. (E); and reproduction factor of L. variegatus (F) after a 28 day exposure to PS microplastic concentrations ranging from 0 to 40% in sediment dw.
Growth
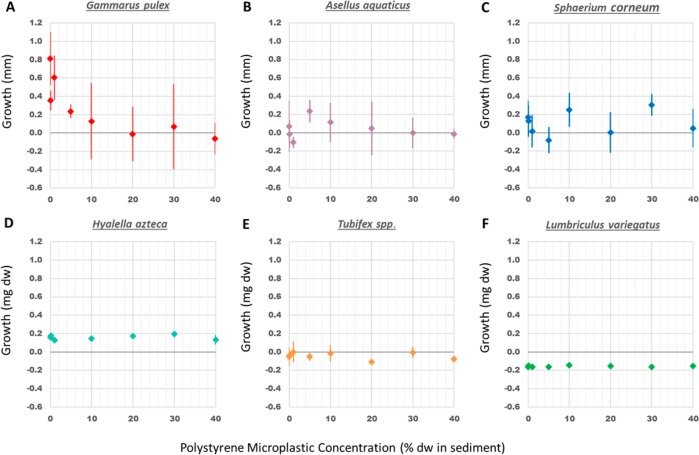
The effect of PS microplastic concentrations on the growth of the organisms was assessed as a difference in length (in mm) for G. pulex, A. aquaticus, and S. corneum, and as a difference in dry weight (in mg) for H. azteca, Tubifex spp. and L. variegatus (Figure 2). One-way ANOVA and regression analysis showed no relation between PS microplastic concentrations in sediment and the growth of A. aquaticus, S. corneum, H. azteca, L. variegatus, and Tubifex spp. However, individuals of G. pulex exposed to sediment containing high microplastic concentrations (from 10 to 40%) showed a significant reduction in size compared to controls (ANOVA, p-value = 0.002). The fit of the log–logistic model (eq 2) was highly significant (p-value = 2.27 × 10–4) and resulted in a EC50 value of 3.57% sediment dw (±3.22) and an EC10 value of 1.07% (SI Figure S3). The rather high uncertainty (reflected through the SD) in the EC50 value reflects the rather high variability among replicates. These outcomes reveal that a chronic exposure to PS microplastic results in a species specific and dose-dependent effect of PS microplastics on the growth of the benthic macroinvertebrates tested. However, while the growth of G. pulex was significantly reduced with increasing PS microplastic dose in sediment, the growth of the five other organisms was not altered by the presence of these particles at concentrations up to 40% plastic in sediment dw. Hence, the EC10 values for these species are higher than 40% plastic in sediment dw. Growth inhibition of G. pulex by a chronic PS microplastics exposure from sediment has not been reported before. However, chronic exposure of a closely related freshwater shrimp, G. fossarum, to poly(methyl methacrylate) (PMMA) and polyhydroxybutyrate (PHB) microplastics in water caused a decrease in growth at a concentration of 100 000 microplastics particles per individual with a similar size range.12H. azteca, another amphipod in the present study, showed no reduction in growth after a 28 day exposure to PS microplastic concentration up to 40% in sediment. In contrast, a previous study showed a decrease in the growth of H. azteca after a 28 day exposure to PE microplastics in water at concentrations of 5000 and 10 000 PE microplastic particles per mL.11 Such differences between study outcomes may relate to (a) differences in the exposure medium, as the presence of natural particles seems to reduce the ingestion of microplastics in freshwater invertebrates,15 and (b) to a higher bioavailability of particles in suspension as compared to particles mixed in the sediment as in the present bioassays. No effects were found on growth for the marine isopod I. emarginata exposed to PE, PS, and PA microplastics in water,16 while weight loss of the marine lugworm A. marina was reported at concentrations of 7.4% PS microplastics18 and >5% uPVC microplastics in sediment dw.17
Figure 2.
Mean growth (±s.d.) as length (in mm) of G. pulex (A), A. aquaticus (B), S. corneum (C); and as dry weight (in mg) of H. azteca (D), Tubifex spp. (E) and L. variegatus (F) after a 28 day exposure to PS microplastic concentrations ranging from 0 to 40% in sediment dw.
Feeding Activity
Feeding rate of G. pulex and H. azteca was calculated as the dry weight (in mg) of Populus sp. leaves consumed per organism per day (SI Figure S4). No differences were found on the feeding activity of G. pulex and H. azteca after a 28 day exposure to PS microplastic concentrations up to 40% in sediment dw (SI Figure S4). These results are in accordance with Weber et al., 2018, where no effects on the feeding activity of G. pulex were found after an exposure to PET microplastics in water.14 These findings indicate that weight loss of G. pulex was probably not caused by a reduction in the consumption of Populus sp. leaves during the experiment and that the presence of microplastics in the sediment did not alter the feeding rate of these benthic amphipods. Similarly, while the growth of G. fossarum was reduced after a 28 day exposure to PMMA and PHB particles, the feeding rate was also unaffected.12
The egestion rate of L. variegatus and Tubifex spp. was assessed as the dry weight of the faeces egested (in mg) per organism per h over a 15 day period (SI Figure S5). At the end of the experiment, all L. variegatus and Tubifex spp. survived and no reproduction was observed in the additional 15 day period experiment. The egestion rate of L. variegatus increased during the first week of exposure and then decreased until the end of experiment, whereas the egestion rate for Tubifex spp. increased over time until the end of experiment (SI Figure S5). The average egestion rates of L. variegatus and Tubifex spp. were 0.43 and 0.32 mg dry faeces per mg dry organism per h, respectively, and this difference was significant along the sampling time (RM ANOVA, p-value < 0.05). However, microplastic exposure had no negative effect on the egestion rate of the worms and the interactive effect between microplastic exposure and sampling time was also not significant.
Ingestion, Retention, and Egestion of Microplastic
At the end of the 28 day exposure to PS microplastics, organisms of G. pulex and H. azteca were allowed to clean their gut for 24 h. Remaining faecal pellets as well as the body of the organisms were checked for microplastics, separately. No microplastics were found in the body nor in the faecal pellets of H. azteca at any concentration, indicating that these organisms did not ingest microplastic particles in the size range of 20–500 μm PS. This is consistent with the lack of effect found for this species in the present 28 day exposure test.
In contrast to H. azteca, PS microplastics were found at all concentrations in the body of G. pulex, as well as in their faecal pellets after a 24 h depuration time. Size frequency distribution of the microplastics found in the body of all organisms (n = 191) ranged from 22 to 165 μm, with an average size of 61 μm (SI Figure S6A). The size frequency distribution of the microplastics found in the faeces (n = 840) ranged from 16 to 165 μm, with an average size of 57 μm (SI Figure S6B). Microplastics with a size >165 μm accounted for only <0.01% of the total amount (in number) and were considered to originate from an external source of microplastics (i.e., particles attached to the external body of the organisms), and were removed from the analysis. The total amount of ingested particles (retained + egested) (n = 1031) ranged from 16 to 165 μm, with an average size of 58 μm (SI Figure S6C).
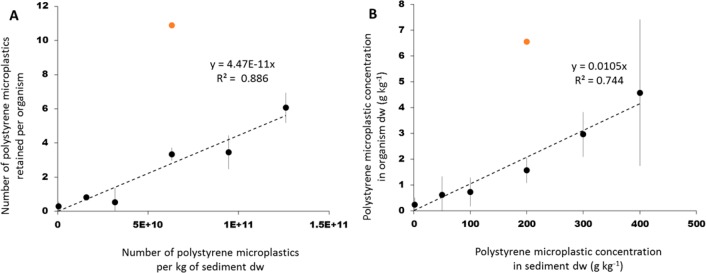
A linear regression revealed a significant, positive relation between the number of microplastics inside the body of G. pulex and the number of microplastics in the sediment exposure medium (linear regression, n = 23, p-value 6.65 × 10–8; Figure 3A). One of the concentrations was designated as an outlier (Iglewics and Hoaglin’s robust test) which was not taken into account in the subsequent determination of the regression parameters. A linear relationship was also found when mass-based concentrations were used (linear regression, n = 23, p-value = 3.97 × 10–7, Figure 3B).
Figure 3.
Mean PS microplastic concentration (n = 4) per individual of G. pulex (±SD) as a function of the PS microplastic concentrations in sediment, as (A) number of PS microplastics retained per organism by number of PS microplastics per kg of sediment dw; (B) g kg–1 of PS microplastics retained per organism dw by g kg–1 of PS microplastics per sediment dw. Linear regressions were based on the individual data points (n = 22) with omission of one suspected outlier (orange marker).
There was also a significant, positive relation between the number of PS microplastics in the faeces egested by G. pulex and the number of PS microplastics in sediment (linear regression, n = 23, p-value 6.63 × 10–06) (SI Figure S7A). Similarly, the weight of PS microplastics egested per organism dw (g kg–1) also increased linearly with the weight of the PS microplastics in sediment dw (g kg–1) (linear regression, n = 23, p-value = 4.9 × 10–07) (SI Figure S7B).
These data show that up to a concentration of 40%, uptake by G. pulex (Figure 3) is proportional to the concentration in the sediment, either expressed as number or as mass. Given the demonstrated proportionality between exposure and uptake, the slope of the line in Figure 3A and B can be interpreted as trophic transfer factors (TTF) with a value of (4.47 ± 0.35) × 10–11 (TTFnumber; Figure 3A) and (10.5 ± 1.3) × 10–3 (TTFmass; Figure 3B). The TTF represents the ratio of the microplastic concentration in the organism and that in the sediment exposure medium, which appears to be constant up to 40% sediment dw. These TTF values are low, which can be explained by the fact that only a limited part of the size range in the sediment is actually taken up, that is, the TTFs mechanistically reflect transfer and size selection. When corrected for the 165–500 μm biounavailable fraction, pure estimates of net transfer are obtained, being TTF = (5.16 ± 0.40) × 10–11 (number) and 0.028 ± 0.0036 (mass). As previously stated for other freshwater amphipods exposed to microplastics, our results indicate that growth reduction of G. pulex was a sublethal effect caused by a lower ability of these organisms to assimilate food due to the ingestion of PS microplastics,11,12 as well as by the gut blockage by these particles due to a longer excretion time needed to depurate their gut.11 Therefore, the observed constancy and magnitude of TTF may still change over time. Based on microplastic excretion studies performed with other freshwater amphipods exposed to different microplastic types,11,12G. pulex is expected to be able to completely depurate if enough time is given and if the ingestion of particles concludes. These findings indicate that microplastic uptake is size-dependent and that shape might affect the ability of organisms to excrete them. This is in accordance with previous studies showing that microplastic uptake by freshwater invertebrates is size- specific and feeding type dependent and that irregularly shaped microplastics need a significant longer clearance time in comparison to spherical microplastics.11,15 Moreover, the high mobility of G. pulex(46) could have increased microplastic uptake in comparison to the other epibenthic species, revealing the importance of species specific traits in the effects of microplastics on benthic invertebrates.
General Discussion
We showed that for a range of freshwater species with different traits exposed to PS microplastics in sediment under the same environmentally relevant conditions, no effect was found for five out of six species even at extremely high concentrations (40% sediment dw). Only for one of the species, G. pulex, a significant reduction in growth was found, which is likely to be explained by the demonstrated size-selective uptake of PS microplastics and their slow excretion, leading to a depletion of energy reserves as found earlier for marine worms as a result of microplastic ingestion.17 As mentioned earlier, our wide range of PS particle sizes and shapes can be considered as a fair approximation of environmental microplastics when it concerns their physical effects. Field measured concentrations in freshwater sediments2−4,7,8 although often provisional due to methodological limitations, are far below the calculated EC10 or EC50 effect threshold values for G. pulex. This means that extrapolating these results to the environment leads to small chances of such physical effects, and consequently low current risk for the benthic community of freshwater systems. However, microplastic concentrations are expected to increase in the environment,47 which implies that effects are not unthinkable in the future. Finding high effects thresholds for most species does not rule out risks on the level of biodiversity or on community functioning, as these also depend on the performance of the most sensitive species, here G. pulex. In fact, G. pulex plays a key role in the processing of coarse particulate organic matter in streams,48 is an important prey for fish,49 and its feeding inhibition has shown to alter the benthic macroinvertebrate community,36 which means that responses at community and ecosystem levels could occur over time. Eventually, the combination of effect threshold data in species sensitivity distributions may represent a more refined approach as part of a higher tier in the assessment of physical effects of microplastics.9 Moreover, for G. pulex we demonstrated ingestion to be proportional to dose and we introduced the concept of TTF accumulation factors for microplastic, which may be useful in exposure assessments. If the observed ingestion behavior would be confirmed to be general among benthic invertebrates, uptake and exposure models may rely on using constant ingestion rates or steady state TTFs for a wide range of microplastic concentrations in sediments.
Acknowledgments
This study was funded by the Dutch Technology Foundation TTW, project no. 13940. We acknowledge additional support from KWR; IMARES; NVWA; RIKILT; the Dutch Ministry of Infrastructure and the Environment; the Dutch Ministry of Health, Welfare and Sport; Wageningen Food & Biobased Research; STOWA; RIWA; and water boards Hoogheemraadschap van Delfland, Zuiderzeeland, Rijn en IJssel, Vechtstromen, Scheldestromen, Aa en Maas, de Dommel, and Rivierenland. We thank John Beijer and Jos Sewalt for their practical assistance and advice, Svenja Mintenig for her help in the development of the micro-FTIR analysis protocol and Lyke Stuurman and Dianneke van Wijk for performing the first pilot tests.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.est.7b05367.
Additional information as noted in the text (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Thompson R. C.; Moore C. J.; vom Saal F. S.; Swan S. H. Plastics, the environment and human health: current consensus and future trends. Philos. Trans. R. Soc., B 2009, 364 (1526), 2153–2166. 10.1098/rstb.2009.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton A. A.; Walton A.; Spurgeon D. J.; Lahive E.; Svendsen C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. 10.1016/j.scitotenv.2017.01.190. [DOI] [PubMed] [Google Scholar]
- Wagner M.; Scherer C.; Alvarez-Muñoz D.; Brennholt N.; Bourrain X.; Buchinger S.; Fries E.; Grosbois C.; Klasmeier J.; Marti T.; et al. Microplastics in freshwater ecosystems: what we know and what we need to know. Environ. Sci. Eur. 2014, 26 (1), 12. 10.1186/s12302-014-0012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eerkes-Medrano D.; Thompson R. C.; Aldridge D. C. Microplastics in freshwater systems: A review of the emerging threats, identification of knowledge gaps and prioritisation of research needs. Water Res. 2015, 75, 63–82. 10.1016/j.watres.2015.02.012. [DOI] [PubMed] [Google Scholar]
- Besseling E.; Quik J. T. K.; Sun M.; Koelmans A. A. Fate of nano- and microplastic in freshwater systems: A modeling study. Environ. Pollut. 2017, 220, 540–548. 10.1016/j.envpol.2016.10.001. [DOI] [PubMed] [Google Scholar]
- Kooi M.; Besseling E.; Kroeze C.; Van Wezel A.; Koelmans A. A.. Modelling the fate and transport of plastic debris in fresh waters. Review and guidance. In Freshwater Microplastics. Emerging Environmental Contaminants?; Wagner M., Lambert S., Eds.; Springer, Hdb Env Chem, 2018, 58. [Google Scholar]
- Klein S.; Worch E.; Knepper T. P. Occurrence and Spatial Distribution of Microplastics in River Shore Sediments of the Rhine-Main Area in Germany. Environ. Sci. Technol. 2015, 49 (10), 6070–6076. 10.1021/acs.est.5b00492. [DOI] [PubMed] [Google Scholar]
- Leslie H. A.; Brandsma S. H.; Van Velzen M. J. M.; Vethaak A. D. Microplastics en route: Field measurements in the Dutch river delta and Amsterdam canals, wastewater treatment plants, North Sea sediments and biota. Environ. Int. 2017, 101, 133–142. 10.1016/j.envint.2017.01.018. [DOI] [PubMed] [Google Scholar]
- Koelmans A. A.; Besseling E.; Foekema E.; Kooi M.; Mintenig S.; Ossendorp B. C.; Redondo-Hasselerharm P. E.; Verschoor A.; van Wezel A. P.; Scheffer M. Risks of Plastic Debris: Unravelling Fact, Opinion, Perception, and Belief. Environ. Sci. Technol. 2017, 51 (20), 11513–11519. 10.1021/acs.est.7b02219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imhof H. K.; Ivleva N. P.; Schmid J.; Niessner R.; Laforsch C. Contamination of beach sediments of a subalpine lake with microplastic particles. Curr. Biol. 2013, 23 (19), R867–R868. 10.1016/j.cub.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Au S. Y.; Bruce T. F.; Bridges W. C.; Klaine S. J. Responses of Hyalella azteca to acute and chronic microplastic exposures. Environ. Toxicol. Chem. 2015, 34 (11), 2564–2572. 10.1002/etc.3093. [DOI] [PubMed] [Google Scholar]
- Straub S.; Hirsch P. E.; Burkhardt-Holm P. Biodegradable and Petroleum-Based Microplastics Do Not Differ in Their Ingestion and Excretion but in Their Biological Effects in a Freshwater Invertebrate Gammarus fossarum. Int. J. Environ. Res. Public Health 2017, 14 (7), 774. 10.3390/ijerph14070774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley R. R.; Woodward J. C.; Rothwell J. J. Ingestion of Microplastics by Freshwater Tubifex Worms. Environ. Sci. Technol. 2017, 51 (21), 12844–12851. 10.1021/acs.est.7b03567. [DOI] [PubMed] [Google Scholar]
- Weber A.; Scherer C.; Brennholt N.; Reifferscheid G.; Wagner M. PET microplastics do not negatively affect the survival, development, metabolism and feeding activity of the freshwater invertebrate Gammarus pulex. Environ. Pollut. 2018, 234, 181–189. 10.1016/j.envpol.2017.11.014. [DOI] [PubMed] [Google Scholar]
- Scherer C.; Brennholt N.; Reifferscheid G.; Wagner M. Feeding type and development drive the ingestion of microplastics by freshwater invertebrates. Sci. Rep. 2017, 7 (1), 17006. 10.1038/s41598-017-17191-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämer J.; Gutow L.; Köhler A.; Saborowski R. Fate of microplastics in the marine isopod Idotea emarginata. Environ. Sci. Technol. 2014, 48 (22), 13451–13458. 10.1021/es501385y. [DOI] [PubMed] [Google Scholar]
- Wright S. L.; Rowe D.; Thompson R. C.; Galloway T. S. Microplastic ingestion decreases energy reserves in marine worms. Curr. Biol. 2013, 23 (23), R1031–R1033. 10.1016/j.cub.2013.10.068. [DOI] [PubMed] [Google Scholar]
- Besseling E.; Wegner A.; Foekema E. M.; Van Den Heuvel-Greve M. J.; Koelmans A. A. Effects of microplastic on fitness and PCB bioaccumulation by the lugworm Arenicola marina (L.). Environ. Sci. Technol. 2013, 47 (1), 593–600. 10.1021/es302763x. [DOI] [PubMed] [Google Scholar]
- Besseling E.; Foekema E. M.; van den Heuvel-Greve M. J.; Koelmans A. A. The Effect of Microplastic on the Uptake of Chemicals by the Lugworm Arenicola marina (L.) under Environmentally Relevant Exposure Conditions. Environ. Sci. Technol. 2017, 51 (15), 8795–8804. 10.1021/acs.est.7b02286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaposi K. L.; Mos B.; Kelaher B. P.; Dworjanyn S. A. Ingestion of Microplastic Has Limited Impact on a Marine Larva. Environ. Sci. Technol. 2014, 48 (3), 1638–1645. 10.1021/es404295e. [DOI] [PubMed] [Google Scholar]
- Lo H. K. A.; Chan K. Y. K. Negative effects of microplastic exposure on growth and development of Crepidula onyx. Environ. Pollut. 2018, 233, 588–595. 10.1016/j.envpol.2017.10.095. [DOI] [PubMed] [Google Scholar]
- Connors K. A.; Dyer S. D.; Belanger S. E. Advancing the quality of environmental microplastic research. Environ. Toxicol. Chem. 2017, 36 (7), 1697–1703. 10.1002/etc.3829. [DOI] [PubMed] [Google Scholar]
- Karami A. Gaps in aquatic toxicological studies of microplastics. Chemosphere 2017, 184, 841–848. 10.1016/j.chemosphere.2017.06.048. [DOI] [PubMed] [Google Scholar]
- Browne M. A.; Niven S. J.; Galloway T. S.; Rowland S. J.; Thompson R. C. Microplastic moves pollutants and additives to worms, reducing functions linked to health and biodiversity. Curr. Biol. 2013, 23 (23), 2388–2392. 10.1016/j.cub.2013.10.012. [DOI] [PubMed] [Google Scholar]
- Andrady A. L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62 (8), 1596–1605. 10.1016/j.marpolbul.2011.05.030. [DOI] [PubMed] [Google Scholar]
- Maltby L. Sensitivity of the crustaceans Gammarus pulex (L.) and Asellus aquaticus (L.) to short-term exposure to hypoxia and unionized ammonia: observations and possible mechanisms. Water Res. 1995, 29 (3), 781–787. 10.1016/0043-1354(94)00231-U. [DOI] [Google Scholar]
- Kupryianchyk D.; Reichman E. P.; Rakowska M. I.; Peeters E. T. H. M.; C Grotenhuis J. T.; Koelmans A. A. Ecotoxicological Effects of Activated Carbon Amendments on Macroinvertebrates in Nonpolluted and Polluted Sediments. Environ. Sci. Technol. 2011, 45 (19), 8567–8574. 10.1021/es2014538. [DOI] [PubMed] [Google Scholar]
- Diepens N. J.; Koelmans A. A.; Baveco H.; van den Brink P. J.; van den Heuvel-Greve M. J.; Brock T. C. M.. Prospective environmental risk assessment for sediment-bound organic chemicals: A proposal for tiered effect assessment. In Reviews of Environmental Contamination and Toxicology; de Voogt P., Ed.; Springer, Cham; 239, 2016; p 77. [DOI] [PubMed] [Google Scholar]
- Rodriguez P.; Martinez-Madrid M.; Arrate J. A.; Navarro E. Selective feeding by the aquatic oligochaete Tubifex tubifex (Tubificidae, Clitellata). Hydrobiologia 2001, 463, 133–140. 10.1023/A:1013199507341. [DOI] [Google Scholar]
- Sidney L. A.; Diepens N. J.; Guo X.; Koelmans A. A. Trait-based modelling of bioaccumulation by freshwater benthic invertebrates. Aquat. Toxicol. 2016, 176, 88–96. 10.1016/j.aquatox.2016.04.017. [DOI] [PubMed] [Google Scholar]
- OECD . OECD Guidelines for the testing of chemicals. Test No. 225: Sediment-Water Lumbriculus Toxicity Test Using Spiked Sediment. OECD Guidel, 2007. [Google Scholar]
- Ankley G. T.; Schubauer-Berigan M. K.; Monson P. D. Influence of pH and hardness on toxicity of ammonia to the amphipod Hyalella azteca. Can. J. Fish. Aquat. Sci. 1995, 52 (10), 2078–2083. 10.1139/f95-801. [DOI] [Google Scholar]
- Schubaur-Berigan M. K.; Monson P. D.; West C. W.; Ankley G. T. Influence of pH on the toxicity of ammonia to Chironomus tentans and Lumbriculus variegatus. Environ. Toxicol. Chem. 1995, 14 (4), 713–717. 10.1897/1552-8618(1995)14[713:IOPOTT]2.0.CO;2. [DOI] [Google Scholar]
- Arrate J. Á.; Rodrigez P.; Martinez-Madrid M. Tubifex tubifex chronic toxicity test using artificial sediment: methodological issues. Limnetica 2014, 23 (1–2), 25–36. [Google Scholar]
- Wilhelm F. M.; Lasenby D. C. Seasonal Trends in the Head Capsule Length and Body Length/Weight Relationships of Two Amphipod Species. Crustaceana 1998, 71 (4), 399–410. 10.1163/156854098X00518. [DOI] [Google Scholar]
- Maltby L.; Clayton S. A.; Wood R. M.; McLoughlin N. Evaluation of the Gammarus pulex in situ feeding assay as a biomonitor of water quality: Robustness, responsiveness, and relevance. Environ. Toxicol. Chem. 2002, 21 (2), 361–368. 10.1002/etc.5620210219. [DOI] [PubMed] [Google Scholar]
- Leppänen M. T.; Kukkonen J. V. K. Factors affecting feeding rate, reproduction and growth of an oligochaete Lumbriculus variegatus (Müller). Hydrobiologia 1998, 377 (1−3), 183–194. 10.1023/A:1003252520704. [DOI] [Google Scholar]
- Rasmussen J. B. Comparison of gut contents and assimilation efficiency of fourth instar larvae of two coexisting chironomids, Chironomus riparius Meigen and Glyptotendipes paripes (Edwards). Can. J. Zool. 1984, 62 (6), 1022–1026. 10.1139/z84-145. [DOI] [Google Scholar]
- Claessens M.; Van Cauwenberghe L.; Vandegehuchte M. B.; Janssen C. R. New techniques for the detection of microplastics in sediments and field collected organisms. Mar. Pollut. Bull. 2013, 70 (1–2), 227–233. 10.1016/j.marpolbul.2013.03.009. [DOI] [PubMed] [Google Scholar]
- Mintenig S.; Bauerlein P.; Koelmans A. A.; Dekker S.; van Wezel A.. Closing the Gap between Small and Smaller: Towards an Analytical Protocol for the Detection of Micro- And Nanoplastic in Freshwater Systems., MICRO 2016 Conference; Lanzarote, Spain, May 25–27, 2016.
- Polymer Database http://polymerdatabase.com/polymers/polystyrene.html (accessed September 18, 2017).
- Franken R. J. M.; Waluto B.; Peeters E. T. H. M.; Gardeniers J. J. P.; Beijer J. A. J.; Scheffer M. Growth of shredders on leaf litter biofilms: the effect of light intensity. Freshwater Biol. 2005, 50 (3), 459–466. 10.1111/j.1365-2427.2005.01333.x. [DOI] [Google Scholar]
- Kerr D. R.; Meador J. P. Modeling dose response using generalized linear models. Environ. Toxicol. Chem. 1996, 15 (3), 395–401. 10.1002/etc.5620150325. [DOI] [Google Scholar]
- Ritz C. Toward a unified approach to dose-response modeling in ecotoxicology. Environ. Toxicol. Chem. 2010, 29 (1), 220–229. 10.1002/etc.7. [DOI] [PubMed] [Google Scholar]
- Draper N. R.; Smith H.. Applied Regression Analysis; Wiley, 1998. [Google Scholar]
- Vadher A. N.; Stubbington R.; Wood P. J. Fine sediment reduces vertical migrations of Gammarus pulex (Crustacea: Amphipoda) in response to surface water loss. Hydrobiologia 2015, 753 (1), 61–71. 10.1007/s10750-015-2193-5. [DOI] [Google Scholar]
- Rochman C. M.; Browne M. A.; Halpern B. S.; Hentschel B. T.; Hoh E.; Karapanagioti H. K.; Rios-Mendoza L. M.; Takada H.; Teh S.; Thompson R. C. Policy: Classify plastic waste as hazardous. Nature 2013, 494 (7436), 169–171. 10.1038/494169a. [DOI] [PubMed] [Google Scholar]
- Kelly D. W.; Dick J. T. A.; Montgomery W. I. The functional role of Gammarus (Crustacea, Amphipoda): shredders, predators, or both?. Hydrobiologia 2002, 485 (1−3), 199–203. 10.1023/A:1021370405349. [DOI] [Google Scholar]
- Macneil C.; A Dick J. T.; Elwood R. W. The Thropic Ecology of Freshwater Gammarus spp. (Crustacea: Amphipoda): Problems and Perspectives Concerning the Functional Feeding Group Concept. Biol. Rev. Cambridge Philos. Soc. 1997, 72 (3), 349–364. 10.1017/S0006323196005038. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.