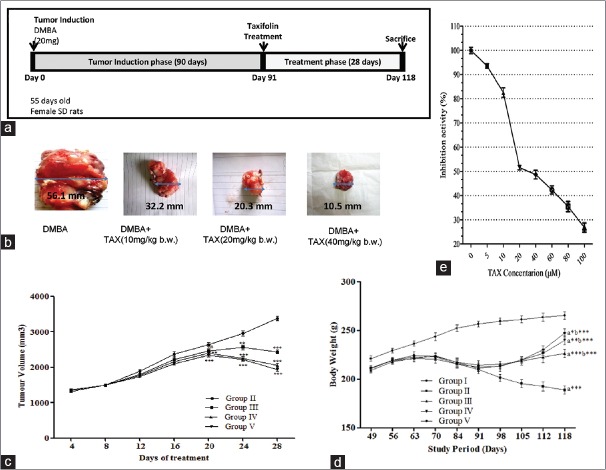
Figure 2.
(a) Treatment protocol for in vivo studies. (b) Tumorigenesis assessment in control and taxifolin-treated experimental animals. Isolated tumor tissue from all cancer bearing treatment groups; (c) tumor volume in all treatment groups. (d) Effect of taxifolin treatment on body weight in cancer-inducing animals. Data represented as mean standard error of the mean (n = 10); where ***P < 0.001; **P < 0.01; *P < 0.05; nsp > 0.05; Group II - 7,12-dimethylbenz(a) anthracene (20 mg); Group III - 7,12-dimethylbenz (a) anthracene (20 mg) + taxifolin (10 mg/kg b. w.); Group IV - 7,12-dimethylbenz (a) anthracene (20 mg) + taxifolin (20 mg/kg b. w.); Group V - 7,12-dimethylbenz(a) anthracene (20 mg) + taxifolin (40 mg/kg b. w.). (e) Effect of TAX on CYP1A1 inhibition in vitro. CYP1A1 activity was determined by a luciferase assay with CYP1A1 substrate. All experiments were performed in triplicate. Bars indicate mean ± standard deviation * P < 0.05 versus the control