Abstract
Introduction:
Jatyadi ghrita is a classical Ayurvedic formulation indicated in the treatment of various types of ulcers.
Aim:
The study was designed to explore the wound healing properties of Jatyadi Ghrita in diabetes - induced rats.
Materials and Methods:
In the present study, diabetes mellitus was induced to 6 to 8-week-old male Wistar rats by injecting streptozotocin cut 65 mg/kg body weight intravenously by 15 min prior to the administration of Nicotinamide at 230 mg/kg body weight intraperitoneally. Animals having diabetes were used for grouping namely, diabetic control (DC), Ghrita control (GC), positive control (PC), i.e., mupirocin HCl, Jatyadi Ghrita treatment and one group of non-DC. Full-thickness excision wound was created and diameter was recorded. Daily clinical observations were recorded. A wound scoring method was developed. Wound diameter and score were recorded on days 1, 2, 3, 5, 7, 9, 12, 14 and 15. Photographs were taken at the same time interval points. Body weight and feed consumption were recorded weekly. Animals were sacrificed at regular intervals to collect the wound area tissue for histopathology analysis. Obtained data was analyzed statistically.
Results and Observation:
It was observed that there was no significant difference in diameter and percent change in wound healing as compared to any control. However, clinical score and histopathological changes in Jatyadi Ghrita group were improved from the second day of the study as compared to control.
Conclusion:
This indicates that the drug has similar wound healing activity as compared to the modern drug mupirocin HCl.
Keywords: Ayurveda, experimental wound scoring, Jatyadi Ghrita, rat
Introduction
Loss in the integrity of normal anatomical structure of skin is called wound. Hence, healing means restoration of normal anatomical structure and function.[1] Healing is a sequence of events, i.e., movement of specialized cells, signals for influx of connective tissue cells and restoration of blood flow. Certain time-dependent changes occur during healing process, i.e., platelets appear immediately, neutrophils appear within 24 h and macrophages appear within 48 h.[2] These cells occupy the wound bed and release healing signals.[3] These signals are called cytokines. Platelet-derived growth factor (PDGF) and transforming growth factor beta (TGF-β) participate in healing process.[4,5] Proteins such as fibroblast and collagen help in the restructuring of the lost integrity of tissues.
Jatyadi Ghrita is a classical Ayurvedic formulation[6] indicated in ulcers in vital points, oozing/weeping ulcers, deep-rooted ulcers, painful ulcer, bleeding ulcer and non healing ulcer. A study of burn wound healing activity of Jatyadi Ghrita and Jatyadi Taila was performed by Dhande et al. (2012).[7] The study concludes that Jatyadi Ghrita and Jatyadi Taila help in reducing the period of epithelialization as compared to silver sulfadiazine. Wound healing activity of only Jatyadi Taila was indicated. Jatyadi Taila caused reduction in wound area as compared to the application of modern topical antimicrobial agent neosporin.[8]
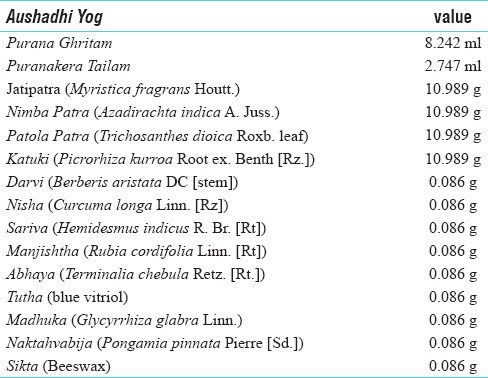
Jatyadi Ghrita contains parts of plants namely, Myristica fragrans Houtt.[9] Azadirachta indica A Juss.[10] Trichosanthes dioica Roxb.[11] Terminalia Chebula Retz.[12] and Hemidesmus indicus R. Br.[13] [Table 1] which are already reported for their wound healing and anti-inflammatory activities. The review of literatures indicated the following basic arenas of exploration for this study:
Table 1.
Ingredients of Jatyadi Ghrita
Whether Jatyadi Ghrita helps in wound healing?
How to evaluate the clinical signs of wound healing in a comparable manner?
Hence, the study was designed to explore the wound healing properties of Jatyadi Ghrita in diabetes-induced rats.
Materials and Methods
Ethical clearance
This study was performed as per the animal ethical guidelines of the Committee for Purpose of Control and Supervision of Experiments on Animals Guidelines for Laboratory Animal Facility after obtaining approval from the Institutional Animal Ethics Committee (IAEC) of National Research Institute of Ayurvedic Drug Development (NRIADD), Kolkata, with ethical approval number IAEC/2014/01 dated 24.04.2014.
Temperature and relative humidity was maintained at 25 ± 1°C and 40%–70%, respectively and illumination was controlled to give approximately a sequence of 12 h light and 12 h dark cycle. Animals were acclimatized for 7 days before initiation of the study. The health examination of the animals was performed by a veterinarian during acclimatization period.
Drug details
Jatyadi Ghrita was obtained from the Arya Vaidya Sala, Kottakkal. The drug contained ingredients as given in classical text as shown in Table 1.[14]
Study design
Eight-week-old Wistar rats were selected based on the body weight and were randomly distributed into four groups in such a way that the means of groups are same and body weight variation is ± 20% of the mean body weight. For gross evaluation group, randomization was done keeping minimum variation in the mean of body weight between the groups before induction of diabetes. Each gross evaluation group contained six male rats and histopathological evaluation group contained seven male rats which were sacrificed at the given time points at days 1, 3, 5, 7, 9, 11 and 15 after wound creation. The five groups for gross and histopathological evaluation were as follows, i.e., negative control (NC), diabetic control (DC), vehicle control (Ghrita) (GC), positive control (mupirocin HCl, trade name-T-Bact, Mfg.- Sanofi) (PC), and Jatyadi Ghrita group (JG). A total of 65 male Wistar rats were obtained from in-house breeding facility of the NRIADD, Kolkata, i.e., 30 for gross evaluation group of which 24 were diabetic and 6 were nondiabetic and for histopathological evaluation group, 35 male Wistar rats were obtained of which 28 were diabetic and 7 were nondiabetic.
Diabetes induction
Diabetes was induced by administering Nicotinamide (Mfg. Himedia Laboratories Pvt., Ltd.) at 230 mg/kg body weight intraperitoneally followed by streptozotocin (Mfg. Himedia Laboratories Pvt., Ltd.) at 65 mg/kg body weight intravenously after 15 min to 8-week-old Wistar rats.[15] Blood glucose levels were evaluated with the help of blood biochemistry analyzer (Robonik Inc.), within the 1st week, after 21 days, before wound creation and at the termination of the study, i.e. before necropsy. Blood glucose levels were evaluated by strip method only to check reversibility of the diabetes whenever desired. The rats showing hyperglycemia (glucose level above 250 mg/dl) were further placed in the respective groups.
Blood was collected through retro-orbital plexus of rats. For glucose estimation, blood collection tubes without anticoagulants were used. The serum was separated and processed.
Wound creation and scoring
Rats were given ether anesthesia for depilation. For wound creation, rats were first tranquilized with Sequil (triflupromazine hydrochloride) and later were given local infiltration of lignocaine as local anesthetic. Hairs in the intrascapular region were removed by shaving with the help of a blade. A small round was drawn on the shaved area and wound was created. Full-thickness excision wound was inflicted on the intrascapular region of the rats. Diameter of the wound was recorded. After wound creation, all the rats were maintained on a thermal pad in order to maintain thermoregulation. Rats were given ad libitum feed and water. Recovery from tranquilization was observed carefully and rats were shifted to cage with blotting paper over the rice husk (sterilized) so as to avoid infection and irritation.
Wound contraction and scoring
Diameter of the wound was recorded on days 1, 2, 3, 5, 7, 9, 12, 14 and 15 and percentage change in Diameter was calculated by the following formula:

Feed consumption was measured daily up to 1st week and thereafter at weekly intervals. The feed consumption was calculated on g/100 g body weight basis.
A unique scoring system based on clinical observations was developed [Table 2].
Table 2.
Wound healing score card

Animals were weighed and sacrificed using CO2 euthanasia followed by exsanguinations and were subjected to a detailed gross pathological examination at termination of the study or on the 16th day of wound creation. The observations were recorded as raw data.
Histopathological scoring
Wound tissue collected was subjected to detailed histopathological processing. Tissue was fixed in formalin, trimmed and processed, i.e. dehydrated in graded alcohol, cleared in xylene, and embedded at 58°C–60°C in paraffin. These tissue blocks were cut at 4–5 μ in thickness and stained with hematoxylin and eosin and finally mounted with digital picture exchange. Slides were then studied under light microscope and photographs were taken. Histopathological evaluation and scoring was done as per the method described by Tkalcević et al. (2009).[16] Granulation tissue was described as follows:
Immature granulation tissue: loose granulation tissue (macrophages, fibroblasts) with emerging vessels
Mature granulation tissue: fibroblasts and sparse extracellular matrix proteins forming layers and vessels running perpendicular
Fibrosis: extracellular matrix proteins (mainly collagen) dominating the granulation tissue, fewer fibroblasts, and vessels.
The score for each wound was given in a semi-quantitative manner with grades of 1–3 for each of the three granulation tissue categories.
Wound bed partially covered with granulation tissue
Thin granulation tissue over the whole wound bed
Thick granulation tissue over the whole wound bed.
The data was analyzed statistically as Groups C (Ghrita control), D (PC, mupirocin hydrochloride), and E (Jatyadi Ghrita) and were statistically compared with Group A (NC) and Group B (DC) in order to find the treatment-related effects. Further, parameters such as feed consumption in g/100 g body weight/day, percentage change in wound area and scores of clinical observations were statistically evaluated as compared to NC and DC.
Results
Gross evaluation group
Diabetes was well established indicating hyperglycemia, asthenia, polyphagia, polydipsia and polyurea in clinical evaluation. No significant difference was observed in body weights of all the groups. No significant alteration in feed consumption was observed in group-wise comparison with controls. On days 2 and 3, there was a significant contraction in NC as compared to DC. On day 2, rats treated with only Ghrita showed significant contraction as compared to DC. However, nonsignificant increase in contraction was observed in groups treated with mupirocin HCl and Jatyadi Ghrita. This contraction was comparable to controls. Jatyadi Ghrita-treated rats showed significantly improved healing as compared to NC and DC on day 7. This indicates that Jatyadi Ghrita has a significant effect on clinical signs such as dryness, borders, thickness and crust formation [Charts 1–3] on day 7 of the wound creation.
Chart 1.
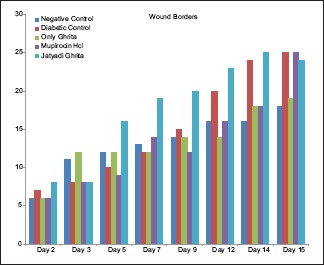
Scores of wound border
Chart 3.
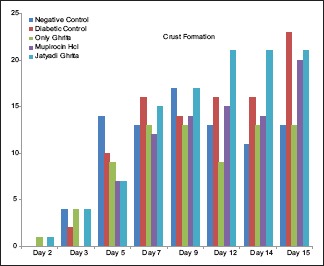
Score of crust formation
Chart 2.
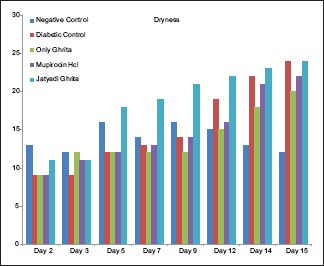
Scores of dryness
Histopathological evaluation group
Contraction and re-epithelialization
No significant difference was noted between histological wound diameter in Jatyadi Ghrita-treated rats and other groups at any point of time, implying that wound contraction rate was similar among all groups. However, the re-epithelialization rate from day 5 onward in Jatyadi Ghrita-treated rats was significantly higher as compared to other groups; this was seen in the form of epidermal hyperplasia at the wound borders. Re-epithelialization was a continuous process that ended 14 to 15 days after wounding.
Granulation tissue
Immature granulation tissue, consisting of macrophages, lymphocytes and plump fibroblasts accompanied by early vascular sprouting within edematous fluid, was well formed and covered almost the whole wound bed in the majority of the Jatyadi Ghrita-treated rats 5 days after the wound creation, whereas in other group of animals, it was just emerging from the wound edges. This implies that initiation of wound healing was faster in Jatyadi Ghrita-treated rats.
By 7 to 9 days after wound creation, the whole wound bed was covered with mature granulation tissue containing fibroblasts and sparse extracellular matrix proteins forming layers and characteristic blood vessels running perpendicular to the directions of the fibroblasts in Jatyadi Ghrita-treated rats. Transformation of “immature” granulation tissue into “mature” granulation tissue was characterized by elongation of fibroblasts, early deposition of collagen fibers and angiogenesis. Whereas, mature granulation tissue appeared in other groups with a delay of 2 days.
The process was completed 12–14 days after wound creation in Jatyadi Ghrita-treated rats with the whole wound bed filled with collagen and scarce inflammatory cells, indicating the appearance of fibrosis [Figures 1–5]. In contrast, in rest of the groups’ rats, 12 days after wound creation, the mature granulation tissue dominated in the wound bed; whereas, deposition of collagen in the extracellular matrix had just begun at the wound edges.
Figure 1.
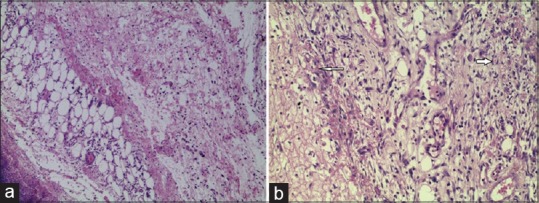
(a) Negative control Day 5: minimal/no immature granulation tissue Deposition as compared to Jatyadi Ghrita group (10 × 10). (b) Jatyadi Ghrita treatment Day 5: Immature granulation tissue with Emerging bloodvessels (angiogenesis) shown in white arrows and fibroblasts and numerous macrophages (10 × 10)
Figure 5.
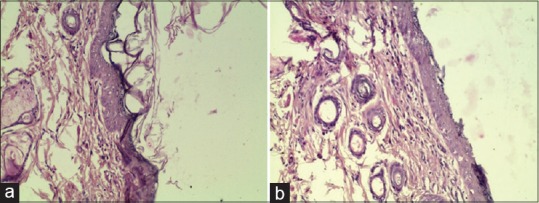
(a) Negative Control Day 15: Re-epithelialization with mild hyperplasia of epidermis and moderate hyperkeratosis (20 × 10). (b) Jatyadi Ghrita Day 15: Re-epithelialization with mild hyperplasia of epidermis (20×10)
Figure 2.
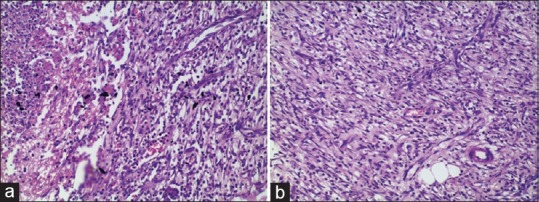
(a) Negative control Day 9: Moderate amount of immature granulation Tissue getting converted to mature (Centre) (20 × 10). (b) Jatyadi Ghrita treatment Day 9: Profound amount of mature granulation Tissue with fibroblasts and sparse extra cellular matrix proteins forming layers, Vesselsrunning perpendicular to the fibroblasts direction (white arrow) (20×10)
Figure 3.
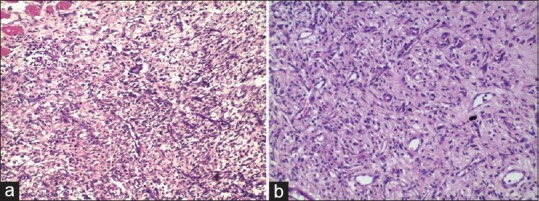
(a) Negative Control Day 11: Profound amount of mature granulation tissue with large number of fibroblasts and scarce extra cellular matrix proteins forming layers as compared to Jatyadi Ghrita treated group, vessels running perpendicular to the fibroblasts direction (20×10). (b) Jatyadi Ghrita treatment Day 11:Profound amount of mature granulation tissue getting converted to fibrosis with increased extra cellular matrix proteins forming layers, vessels running perpendicular to the fibroblasts direction and presence of fibrocytes (20×10)
Figure 4.
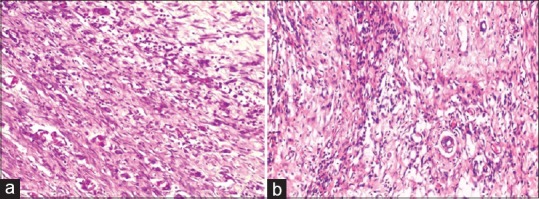
(a) Negative Control Day 15: Fibrosis in progress with moderate amount increased extracellular matrix proteins; however fibroblastsand vessels are still present. Fibrocytes are not properly rearranged and running parallel to each other as compared to Jatyadi Ghrita treatment group (20×10). (b) Jatyadi Ghrita Day 15: Fibrosis in progress with increased extracellular matrix proteins (mainly collagen) dominating, fewer fibroblasts and vessels. Fibrocytes are rearranged and running parallel to each other (20×10)
Inflammation
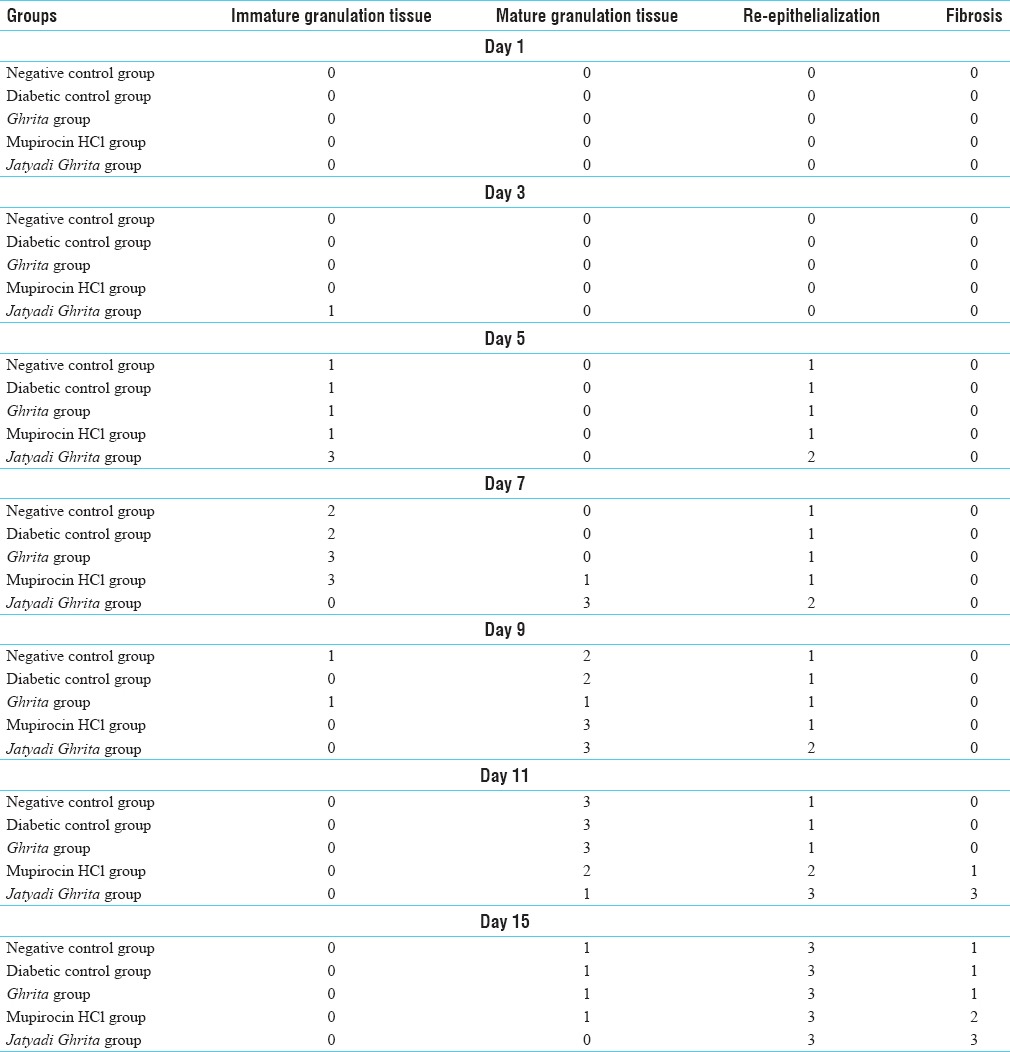
Acute inflammatory changes including neutrophil infiltration, necrosis, exudate formation, fibrin deposition and scab formation were seen in the first 3 days in all the groups’ rats [Table 3]. However, it was comparatively lesser in Jatyadi Ghrita-treated rats and mupirocin -treated rats from 5th days onward but present in rest of the groups till 7 days.
Table 3.
Histopathological grading score for various histomorphological changes
Discussion
Alteration in the normal repair process causes delayed wound healing, i.e., chronic wound or ulcer. Pathologic responses leading to fibrosis or chronic ulcers may occur if any part of the healing sequence is disturbed.[2] Delayed wound healing is characterized by excessive infiltration of neutrophils. Neutrophils release a significant amount of enzymes such as collagenase (i.e., matrix metalloproteinase) that is responsible for the destruction of connective tissue matrix.[17,18] Neutrophils also release elastase that is capable of destroying PDGF and TGF-β.[19] This destruction of cytokines delays the healing process.
Wound healing properties of Jatyadi Ghrita in diabetes-induced rats were explored in the present study. This study was mainly of exploratory design, hence appropriate controls were used for comparison namely, nondiabetic NC, DC, vehicle control and PC, i.e., mupirocin hydrochloride. Percent are decrease in wound diameter/area, feed consumption, clinical observations and histopathological alterations were mainly studied. The important outcome of the study is that Jatyadi Ghrita helps in healing of wound comparable to mupirocin hydrochloride.
The present study reports suitability of streptozotocin–nicotinamide model for any study on diabetes. Food intake was normal in Jatyadi Ghrita-treated group which may be due to palliative effect.
Full-thickness excision model was used for the evaluation of Jatyadi Ghrita. This model was chosen as it accommodates the broadest assessment of the histomorphological events involved in wound healing, including epithelialization, granulation and angiogenesis.[20] In addition, this model has an advantage of providing large area as medications are can be applied directly to the wound bed.[21,22] This model encompasses all skin components, i.e., epidermis, dermis, hypodermis and blood vessels.[23]
There are two distinct variations in clinical wound in humans and experimental wound in laboratory rats: In rats, healing is by contraction and in humans healing is mainly by re-epithelialization.[24,25] Second, in experimental wound, it is possible to note temporal changes in size and shape in all the rats at a time. This helps in deciding the definitive score to each wound and hence to each group. Clinical observations of wound healing were given appropriate numeric scores. The present study indicated that Jatyadi Ghrita does not have significant effect on wound contraction but it improves the healing score for dryness of the wound, decreases the inflammation and helps in the enhancement of crust formation. Histopathological observations indicated that re-epithelialization rate from day 5 onward in Jatyadi Ghrita-treated rats was significantly higher as compared to other groups. Jatyadi Ghrita also indicated faster maturation of granulation tissue, lesser inflammatory cells and early angiogenesis. The present study concludes that Jatyadi Ghrita has analogous wound healing property as that of mupirocin hydrochloride.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Lazarus GS, Cooper DM, Knighton DR, Margolis DJ, Pecoraro RE, Rodeheaver G, et al. Definitions and guidelines for assessment of wounds and evaluation of healing. Arch Dermatol. 1994;130:489–93. [PubMed] [Google Scholar]
- 2.Diegelmann RF, Evans MC. Wound healing: An overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283–9. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- 3.Lawrence WT, Diegelmann RF. Growth factors in wound healing. Clin Dermatol. 1994;12:157–69. doi: 10.1016/0738-081x(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 4.Kim WJ, Gittes GK, Longaker MT. Signal transduction in wound pharmacology. Arch Pharm Res. 1998;21:487–95. doi: 10.1007/BF02975363. [DOI] [PubMed] [Google Scholar]
- 5.Gillitzer R, Goebeler M. Chemokines in cutaneous wound healing. J Leukoc Biol. 2001;69:513–21. [PubMed] [Google Scholar]
- 6.Anonymus. Ayurvedic Pharmacopoeia of India, Part I. Second Edition. New Delhi: Controller of Publication, Civil Lines; 2003. [Google Scholar]
- 7.Dhande Priti P, Simpy R, Kureshee Nargis I, Sanghavi Dhara R, Pandit Vijaya A. Burn wound healing potential of Jatyadi formulations in rats. RJPBCS. 2012;3:746–54. [Google Scholar]
- 8.Shailajan S, Menon S, Pednekar S, Singh A. Wound healing efficacy of Jatyadi Taila: In vivo evaluation in rat using excision wound model. J Ethnopharmacol. 2011;138:99–104. doi: 10.1016/j.jep.2011.08.050. [DOI] [PubMed] [Google Scholar]
- 9.Farzaei MH, Shams-Ardekani MR, Abbasabadi Z, Rahimi R. Scientific evaluation of Edible fruits and spices used for the treatment of peptic ulcer in traditional Iranian medicine. ISRN Gastroenterol. 2013;2013:136932. doi: 10.1155/2013/136932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osunwoke Emeka A, Olotu Emamoke J, Allison Theodore A, Onyekwere Julius C. The wound healing effects of aqueous leave extracts of Azadirachta indica on Wistar rats. J Nat Sci Res. 2013;3:181. [Google Scholar]
- 11.Shivhare Y, Singour PK, Patil UK, Pawar RS. Wound healing potential of methanolic extract of Trichosanthes dioica Roxb (fruits) in rats. J Ethnopharmacol. 2010;127:614–9. doi: 10.1016/j.jep.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 12.Gupta PC. Biological and pharmacological properties of Terminalia chebula Retz. (Haritaki) – An overview. Int J Pharm Pharm Sci. 2012;4(Suppl 3):62–8. [Google Scholar]
- 13.Ganesan S, Parasuraman S, Maheswaran SU, Gnanasekar N. Wound healing activity of Hemidesmus indicus formulation. J Pharmacol Pharmacother. 2012;3:66–7. doi: 10.4103/0976-500X.92516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paradkar H.S, editor. Ashtanga Hridayam of Vagbhatta, Uttarsthana, Adhyaya 25, Ver. 67. Varanasi: Chaukhambha Orientalia; 1982. [Google Scholar]
- 15.Masiello P, Broca C, Gross R, Roye M, Manteghetti M, Hillaire-Buys D, et al. Experimental NIDDM: Development of a new model in adult rats administered streptozotocin and nicotinamide. Diabetes. 1998;47:224–9. doi: 10.2337/diab.47.2.224. [DOI] [PubMed] [Google Scholar]
- 16.Tkalcević VI, Cuzić S, Parnham MJ, Pasalić I, Brajsa K. Differential evaluation of excisional non-occluded wound healing in db/db mice. Toxicol Pathol. 2009;37:183–92. doi: 10.1177/0192623308329280. [DOI] [PubMed] [Google Scholar]
- 17.Nwomeh BC, Liang HX, Cohen IK, Yager DR. MMP-8 is the predominant collagenase in healing wounds and nonhealing ulcers. J Surg Res. 1999;81:189–95. doi: 10.1006/jsre.1998.5495. [DOI] [PubMed] [Google Scholar]
- 18.Nwomeh BC, Liang HX, Diegelmann RF, Cohen IK, Yager DR. Dynamics of the matrix metalloproteinases MMP-1 and MMP-8 in acute open human dermal wounds. Wound Repair Regen. 1998;6:127–34. doi: 10.1046/j.1524-475x.1998.60206.x. [DOI] [PubMed] [Google Scholar]
- 19.Yager DR, Zhang LY, Liang HX, Diegelmann RF, Cohen IK. Wound fluids from human pressure ulcers contain elevated matrix metalloproteinase levels and activity compared to surgical wound fluids. J Invest Dermatol. 1996;107:743–8. doi: 10.1111/1523-1747.ep12365637. [DOI] [PubMed] [Google Scholar]
- 20.Wong VW, Sorkin M, Glotzbach JP, Longaker MT, Gurtner GC. Surgical approaches to create murine models of human wound healing. J Biomed Biotechnol. 2011;2011:969618. doi: 10.1155/2011/969618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toker S, Gulcan E, Cayc MK, Olgun EG, Erbilen E, Ozay Y, et al. Topical atorvastatin in the treatment of diabetic wounds. Am J Med Sci. 2009;338:201–4. doi: 10.1097/MAJ.0b013e3181aaf209. [DOI] [PubMed] [Google Scholar]
- 22.Tsuboi R, Rifkin DB. Recombinant basic fibroblast growth factor stimulates wound healing in healing-impaired db/db mice. J Exp Med. 1990;172:245–51. doi: 10.1084/jem.172.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braiman-Wiksman L, Solomonik I, Spira R, Tennenbaum T. Novel insights into wound healing sequence of events. Toxicol Pathol. 2007;35:767–79. doi: 10.1080/01926230701584189. [DOI] [PubMed] [Google Scholar]
- 24.Falanga V, Saap LJ, Ozonoff A. Wound bed score and its correlation with healing of chronic wounds. Dermatol Ther. 2006;19:383–90. doi: 10.1111/j.1529-8019.2006.00096.x. [DOI] [PubMed] [Google Scholar]
- 25.Galiano RD, Michaels J, 5th, Dobryansky M, Levine JP, Gurtner GC. Quantitative and reproducible murine model of excisional wound healing. Wound Repair Regen. 2004;12:485–92. doi: 10.1111/j.1067-1927.2004.12404.x. [DOI] [PubMed] [Google Scholar]


