Extended Data Figure 7. Nitrate-induced NLP phosphorylation.
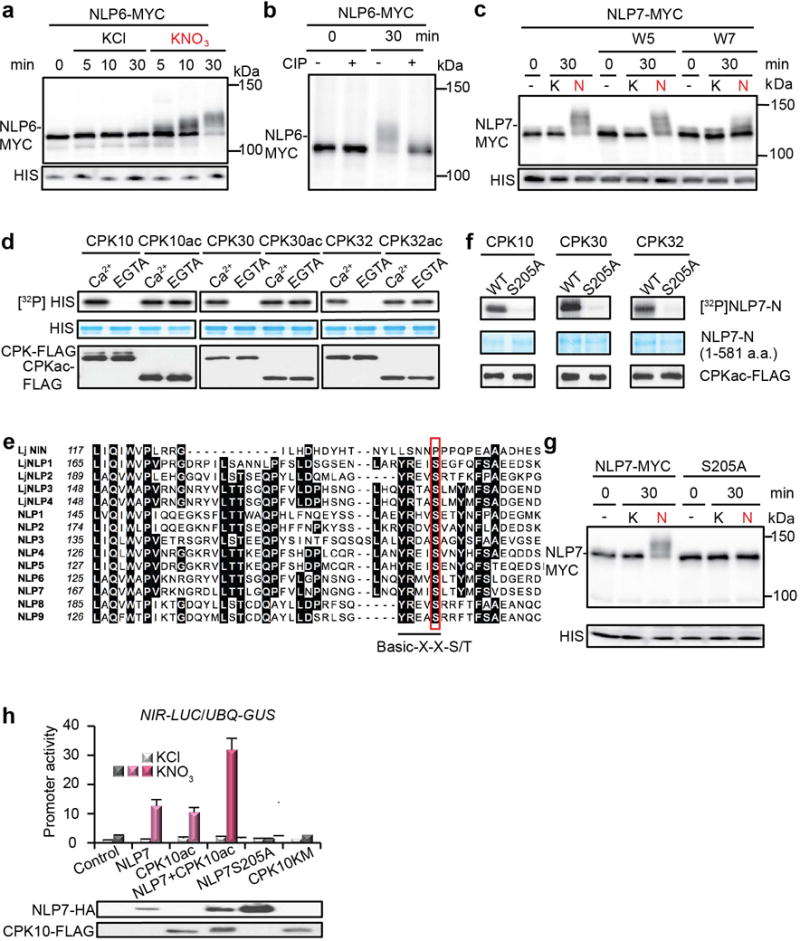
a, Nitrate-induced mobility shift of MYC-tagged NLP6. Transgenic seedlings expressing MYC-tagged NLP6 were grown in liquid medium containing 0.5 mM ammonium succinate as a sole nitrogen source for 4 days and then treated with 10 mM KCl or KNO3 for indicated periods. Immunoblot analysis was carried out with proteins extracted from the seedlings using anti-MYC (MYC) and anti-histone H3 (HIS) antibodies. b, Effect of alkaline phosphatase treatment on mobility shift of MYC-tagged NLP6. Proteins from seedlings treated with 10 mM KNO3 for 0 or 30 min were subjected to calf-intestinal alkaline phosphatase (CIP) treatment. The experiments were repeated twice with consistent results. c, An antagonist of Ca2+ sensors (W7) diminished nitrate-triggered phosphorylation of NLP7. d, Phosphorylation of histone by CPK10/30/32 is Ca2+-dependent. e. Alignment of the amino acid sequences around the conserved CPK phosphorylation site in all NLPs from Arabidopsis thaliana and Lotus japonicus. Conserved amino acid residues are indicated by black boxes. The CPK phosphorylation motif indicated by an underline was identified by multiple web-based bioinformatics tools and literature analysis62-67 with a candidate serine (S205 in NLP7) that is uniquely conserved in nine Arabidopsis NLPs and four orthologous Lotus japonicus NLPs (outlined in red), but not in LjNIN (NODULE INCEPTION). LjNIN, a variant of NLP, which evolved specifically for symbiotic nitrogen fixation in legumes, lacks CPK phosphorylation site. f, S205 in NLP7 is the CPK10/30/32 phosphorylation site. g, Nitrate-induced mobility shift was abolished for NLP7(S205A). h, NLP7 and CPK10ac overexpression showed similar synergism with nitrate for NIR-LUC activation in protoplast transient assays. NLP7 or CPK10ac alone was not effective to enhance NIR-LUC expression without nitrate. CPK10KM lacking kinase activity and NLP7(S205A) lacking the CPK10,30,32 phosphorylation site served as negative controls. NLP7 or CPK10ac protein expression was detected by immunoblot analyses before dividing protoplasts equally and treated with 0.5 mM KCl or KNO3 for 2 h. UBQ10-GUS is a transient expression control. Error bars, s.d., n=5 biological replicates.
