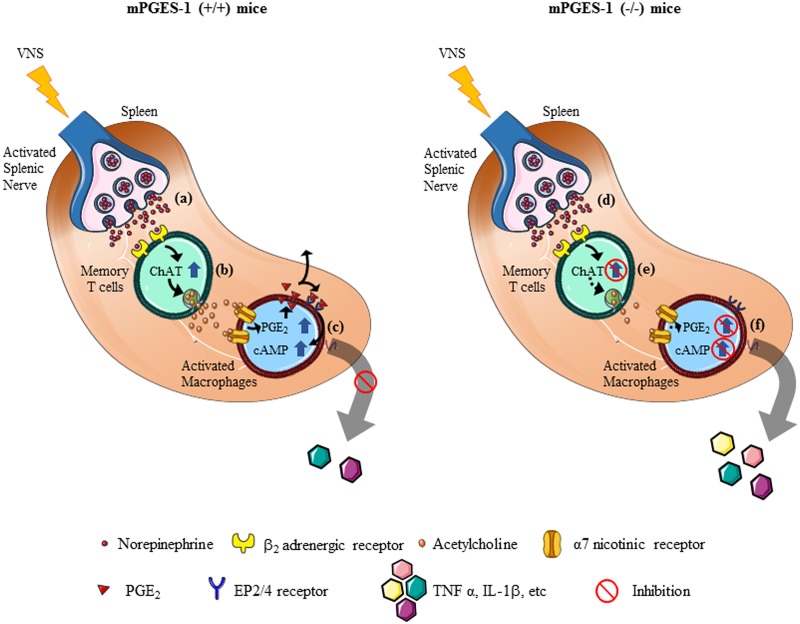
Fig 8. Schematic representation of neuro-immune circuit in the mouse spleen in response to VNS.
In an endotoxaemic mPGES-1(+/+) mouse model, VNS induce norepinephrine (NE) release from the splenic nerve which is in close proximity to the CD4+ CD44hi Cd62Llo T cells (CD4+ memory T cells) (a). NE, thus released, binds to its β2 adrenergic receptor (AR) and stimulates choline acetyltransferase (ChAT) expression in the effector cell (CD4+ memory T cells) leading to release acetylcholine (ACh) (b). ACh- α7 nicotinic receptor interaction on macrophages increases endogenous PGE2 synthesis. Subsequent activation of PGE2 receptors EP2/4 causes cAMP upregulation and inhibits cytokine release, thereby controlling inflammation (c). In our current study, we illustrate that in mice with mPGES-1 genetic deletion (mPGES-1 (-/-)), VNS induced sympathetic SN NE release (d) and activation and β2AR expression on effector memory cells is intact (e). However, other VNS related molecular events such as choline acetyltransferase (ChAT) dependent ACh release (e) and inhibition of cytokines in response to nicotine (α7nAChR agonist) are impaired (f), thereby clearly demonstrating the role of mPGES-1 dependent PGE2 as a crucial mediator in the cholinergic processes related to VNS.