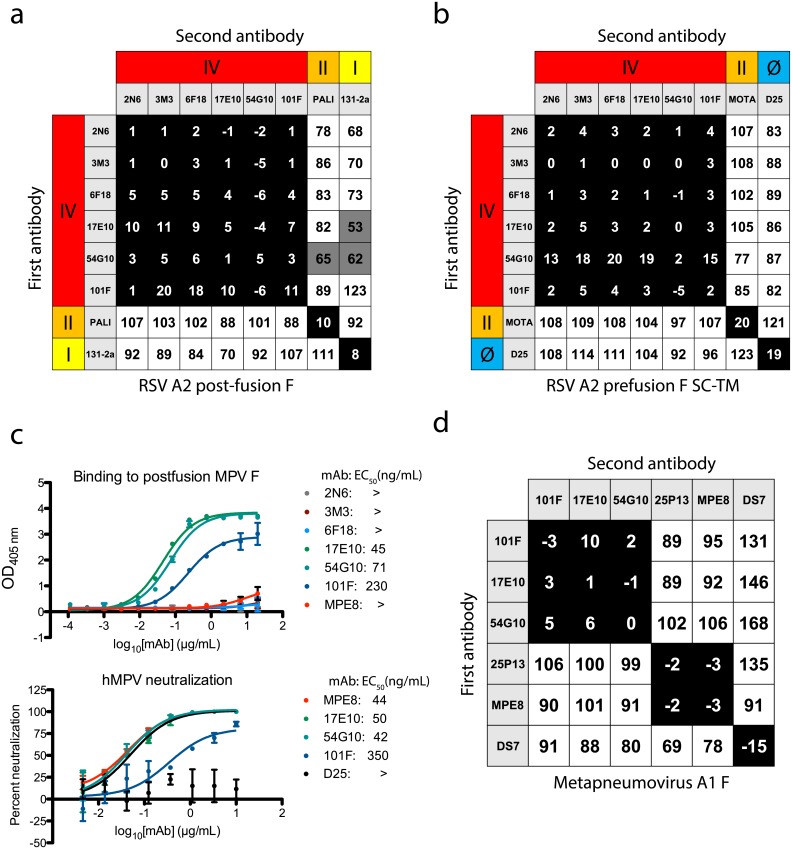
Fig 1. Epitope binning and hMPV F cross-reactivity.
(A) Epitope binning with the newly generated mAbs on post-fusion RSV A2 F protein revealed the mAbs competed for binding to antigenic site IV with the previously described mAbs 101F and 54G10. (B) Epitope binning on pre-fusion RSV A2 F SC-TM suggested binding at antigenic site IV, as competition was not observed with site II mAb motavizumab nor site Ø mAb D25. (C) Binding and neutralization curves indicate mAb 17E10 cross-reacts with hMPV F. The top graph displays ELISA binding data with post-fusion hMPV A1 F protein. Only cross-reactive mAbs 17E10, 54G10, 101F, and MPE8 show binding to the protein, while the site IV mAbs 2N6, 3M3, and 6F18 show no binding. Each data point is the average of three independent experiments, each with four technical replicates. Error bars represent the standard deviation. The bottom graph show neutralization for the cross-reactive mAbs, with D25 used as a negative control. MAbs 17E10, MPE8, and 101F neutralize hMPV F while the D25 control shows no reduction in virus. Data points are the average of three technical replicates, and error bars indicate the standard deviation. (D) Epitope binning using post-fusion hMPV F. MAbs 101F, 17E10, and 54G10 display a similar competition binding pattern to that observed with RSV F protein. The mAbs compete for a site unique from site III mAbs 25P13 and MPE8, and DS7. For epitope binning, data indicate the percent binding of the second antibody in the presence of the first antibody, compared with the second antibody alone. Cells filled in black indicate full competition, in which ≤33% of the uncompeted signal was observed, intermediate competition (gray) if signal was between 33% and 66%, and noncompeting (white) if signal was ≥66%. Antigenic sites are highlighted at the top and side based on competition-binding with the control mAbs D25 (site Ø), 131-2a (site I), palivizumab (PALI) or motavizumab (MOTA) (site II), or 101F (site IV).