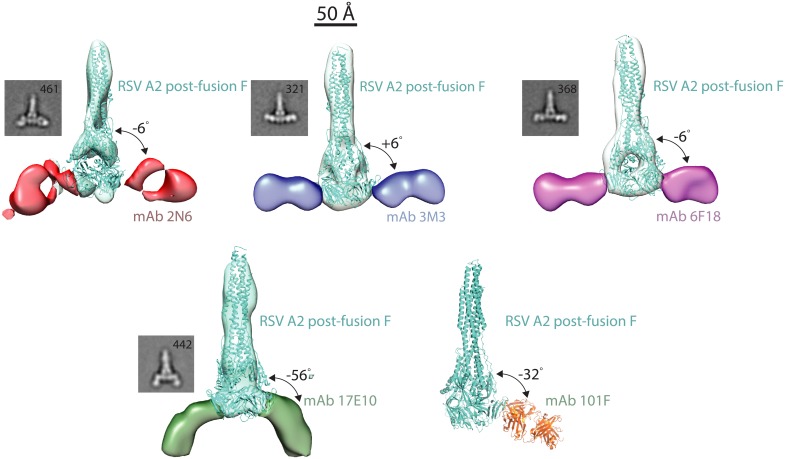
Fig 4. Multiple binding modes at antigenic site IV.
Each site IV mAb was complexed with RSV A2 post-fusion F, and negative-strain electron microscopy images were generated using random-conical tilt analysis. MAbs 3M3 and 6F18 share a similar binding mode, while mAb 2N6 binds antigenic site IV at an angle allowing bypass of the Arg429 residue. MAb 17E10 binds to RSV F at an angle 42° from the plane of the other site IV mAbs. This unique binding pose likely mediates cross-reactivity with hMPV. 3-D reconstructions are displayed for each mAb-RSV F complex, and 2D class averages are displayed below the reconstructions. The X-ray crystal structure of the 101F-peptide complex (PDB ID: 3O41) was aligned to the antigenic site IV region on the post-fusion RSV F crystal structure (3RRR).