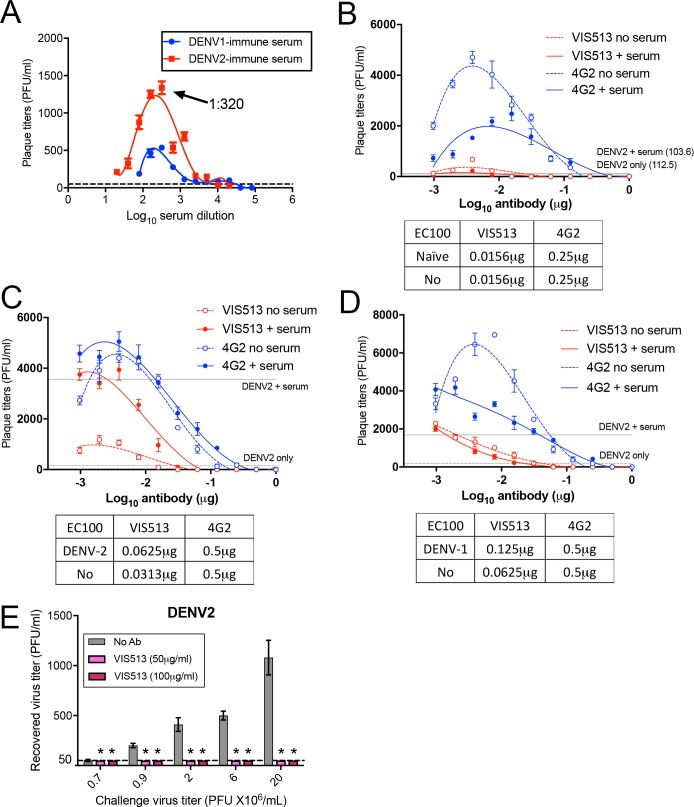
Fig 1. Neutralization of DENV by VIS513 under various conditions in vitro.
A. Infectious titers of DENV2 in the culture supernatant of THP-1.2S incubated with DENV2 (moi 10) reacted with serial two-fold dilutions of convalescent primary DENV1 (blue) and DENV2 (red) sera. Serum dilution of 1:320 resulting in peak enhancement of DENV2 infectious titers is indicated by arrow. Dotted line indicates infectious titers following DENV2 only infection in THP-1.2S. B-D. Infectious titers of DENV2 in THP-1.2S cells following VIS513 or 4G2-mediated neutralization of DENV2 incubated with naïve serum at 1:320 dilution, or in the absence of serum (B). Infectious titers were also measured following VIS513 or 4G2-mediated neutralization of DENV2 incubated with convalescent primary DENV2 (C) and primary DENV1 serum (D) at 1:320 dilution, or in the absence of serum. Dotted lines represent infectious titers following DENV2 infection in the presence or absence of serum, when assay was performed in the absence of VIS513 or 4G2. E. Neutralization efficiency of 50μg/mL or 100μg/mL of VIS513 against different titers of DENV2. Virus incubated without antibody served as control. Dotted line indicates limit of detection for plaque assay. Data are represented as mean ± SEM from two independent experiments.