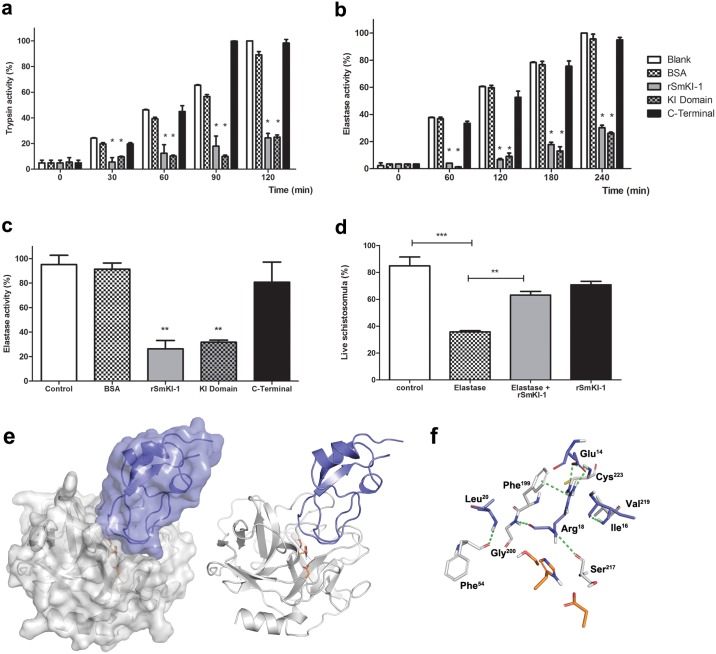
Fig 2. Recombinant SmKI-1 and its Kunitz domain inhibit serine proteases and protect S. mansoni against neutrophil elastase.
Recombinant SmKI-1, its Kunitz or C-terminal domains (100 nM) were tested as inhibitor of serino proteases: (a) Bovine Trypsin activity (100nM), (b) Human Neutrophil Elastase activity (300nM) and (c) Neutrophil-secreted elastase activity. In all experiments, bovine serum albumin (BSA, 300nM) was used as a negative control. Enzyme inhibition was detected over two-hour incubation with rSmKI-1 or its Kunitz domain. Bars indicate each enzyme activity mean ± standard deviation. (d) Protective effect of rSmKI-1 (0.15 mg/mL) in cultured schistosomula treated with purified elastase (0.05 mg/mL). Bars represent live parasites ± standard deviation. Data are representative of at least three independent experiments. For (a) and (b), an asterisk indicate statistically significant differences of rSmKI-1 or Kunitz domain compared to control group p< 0.05. For (c) and (d), ** asterisks indicate statistically significant differences of rSmKI-1, compared to control group or elastase group p< 0.005. (e) Binding mode of SmKI-1 Kunitz domain (purple) to neutrophil elastase (gray) predicted by docking with CLUSPRO 2.0. Residues from elastase catalytic triad (His70, Asp117 and Ser202) are highlighted in orange sticks. (f) Detailed analysis of the docking predicted interface reveals residues involved in hydrogen bonds, a salt bridge and a π-stacking interaction (all interactions shown as green dashes). SmKI-1 residues are represented and labeled in purple, NE residues in gray.