Abstract
Recently, we showed that HIV-1 is sequestered, i.e., trapped, in the intracellular vesicles of oral and genital epithelial cells. Here, we investigated the mechanisms of HIV-1 sequestration in vesicles of polarized tonsil, foreskin and cervical epithelial cells. HIV-1 internalization into epithelial cells is initiated by multiple entry pathways, including clathrin-, caveolin/lipid raft-associated endocytosis and macropinocytosis. Inhibition of HIV-1 attachment to galactosylceramide and heparan sulfate proteoglycans, and virus endocytosis and macropinocytosis reduced HIV-1 sequestration by 30–40%. T-cell immunoglobulin and mucin domain 1 (TIM-1) were expressed on the apical surface of polarized tonsil, cervical and foreskin epithelial cells. However, TIM-1-associated HIV-1 macropinocytosis and sequestration were detected mostly in tonsil epithelial cells. Sequestered HIV-1 was resistant to trypsin, pronase, and soluble CD4, indicating that the sequestered virus was intracellular. Inhibition of HIV-1 intraepithelial sequestration and elimination of vesicles containing virus in the mucosal epithelium may help in the prevention of HIV-1 mucosal transmission.
Keywords: HIV, Epithelial cells, Internalization, Macropinocytosis, Sequestration
1. Introduction
Mucosal epithelia are the first sites of contact between HIV-1 and the human body during the course of infection, and they play a critical role in determining the success of HIV-1 in establishing systemic infection. It has been shown in primate models that application of HIV-1 to the surfaces of intact oropharyngeal (Joag et al., 1997), anal/rectal (Bosch et al., 1997), cervicovaginal and foreskin/penile (Carias et al., 2013; Dinh et al., 2015; Girard et al., 1998; Joag et al., 1997) epithelia can lead to systemic infection of HIV-susceptible immune cells. Application of simian immunodeficiency virus to undamaged oral and vaginal mucosal epithelia also results in the transmigration of simian immunodeficiency virus across these epithelia (Miller et al., 2005; Milush et al., 2004; Stahl-Hennig et al., 1999). Similarly, application of HIV-1 to human foreskin, vaginal and cervical tissue explants ex vivo leads to the transmission of HIV-1 across these epithelia (Carias et al., 2013; Dinh et al., 2015; Ganor et al.; Hladik et al., 2007; Maher et al., 2005; Stoddard et al., 2009; Zhou et al., 2011). Furthermore, interaction of HIV-1 with the mucosal surface of oropharyngeal tissue explants of the fetus or infant leads to infection of CD4+ T lymphocytes, Langerhans/dendritic cells and macrophages, which is critical for HIV-1 mother-to-child transmission (MTCT) (Tugizov et al., 2012). None of these studies showed epithelial infection with HIV-1, indicating that the virus can migrate across intact mucosal epithelia without infecting them.
Recently, we showed that the majority of infectious virions internalized in tonsil, cervical and foreskin epithelial cells do not cross the epithelium but rather are sequestered in their vesicular/endosomal compartments for several days (Yasen et al., 2017). The interaction of activated lymphocytes with epithelial cells containing HIV-1 facilitates the release of virus and its spread from epithelia into lymphocytes. In the present study we investigated the mechanism of HIV-1 sequestration in endosomes of mucosal epithelial cells.
Mucosal epithelial cell surface proteins, including galactosylceramide (GalCer) and heparan sulfate proteoglycans (HSPG), facilitate HIV-1 internalization into epithelial cells (Bobardt et al., 2007; Bomsel and Alfsen, 2003; Fantini et al., 1997; Herrera et al., 2016; Tugizov et al., 2011). HIV-1 internalization into epithelial cells can occur by endocytosis (Bobardt et al., 2007; Daecke et al., 2005; Herrera et al., 2016; Miyauchi et al., 2009; Tugizov et al., 2012; van den Berg et al., 2014; Vidricaire and Tremblay, 2007). HIV-1 internalization in endothelial cells is mediated by macropinocytosis (Liu et al., 2002). Endocytosis could be due to clathrin-, caveolin- and/or lipid raft-associated mechanisms (Mercer et al.). Macropinocytosis is an actin-dependent process induced by membrane ruffling and the formation of large vacuoles, i.e., macropinosomes (Mercer and Helenius, 2009; Tugizov et al., 2013b).
Macropinocytosis plays a critical role in the uptake of viruses belonging to various families, including poxvirus, adeno, and picorna (Mercer and Helenius, 2008, 2009; Mercer et al., 2010; Schelhaas, 2010). Binding of viral envelope-associated phosphatidylserine (PS) to its receptor T-cell immunoglobulin and mucin domain 1 (TIM-1) triggers macropinocytosis (Mercer and Helenius, 2008; Mercer et al., 2010; Shiratsuchi et al., 2000). The outer leaflet of the HIV-1 envelope contains PS (Aloia et al., 1988, 1993b; Callahan et al., 2003; Gekonge et al., 2006), and HIV-1-associated PS facilitates the viral infection of macrophages (Callahan et al., 2003). TIM-1 also promotes HIV-1 entry into CD4+ T lymphocytes (Li et al., 2014). However, the interaction of TIM-1 with HIV-PS during the release of progeny virions inhibits virus release, retaining viral particles to the cell surface (Li et al., 2014). Expression of TIM-1 has been shown in epithelia of oral, lung, cornea, conjunctiva, and kidney (Freeman et al., 2010; Ichimura et al., 2008, 1998; Kondratowicz et al., 2011; Setty and Betal, 2008).
Virus internalized by endocytosis and macropinocytosis may follow intracellular trafficking pathways via early and late endosomes (Mercer and Helenius, 2009; Mercer et al.). Macropinosomes may also fuse with each other and form large vacuoles, which may exist independently from the early and late endosomes (Araki et al., 2006; Falcone et al., 2006; Hamasaki et al., 2004; Hewlett et al., 1994; Racoosin and Swanson, 1993). Early endosomes may serve as sorting compartments, regulating the delivery of internalized virus to various destinations by transcytosis and/or recycling (Jovic et al., 2010; Tuma and Hubbard, 2003). Early endosomal compartments have tubular and vacuolar domains, and the vacuolar domains mature into late endosomes (Huotari and Helenius, 2011), leading to formation of multivesicular bodies (MVB) and lysosomes (Dobrowolski and De Robertis, 2012; Hanson and Cashikar, 2012). MVBs contain a network of intraluminal vesicles. The main function of MVB is delivering cargo into lysosomes, where it is degraded (Fader and Colombo, 2009; Piper and Katzmann, 2007). However, MVB compartments can also serve as storage for internalized and recycled proteins, including cell surface receptors and ligands (Cullen and Korswagen, 2012; Kerr et al., 2006; Piper and Katzmann, 2007; Uchil and Mothes, 2005; Vieira et al., 2014). MVB can fuse with plasma membranes, releasing cargo into the extracellular environment (Nickerson et al., 2006; Piper et al., 2014; Piper and Luzio, 2007). The formation of MVB is initiated by fission of early endosomal compartments from their cytoplasmic face, generating a new compartment with intraluminal vesicles (McCullough et al., 2013; Schmidt and Teis, 2012). The establishment and maturation of MVB are mediated by the coordinated effort of more than 30 proteins, which together are called the “endosomal sorting complexes required for transport” (ESCRT) (Schmidt and Teis, 2012). The ESCRT consist of ESCRT-0, ESCRT-I, and ESCRT-II, which are involved in cargo sorting and membrane invagination (McCullough et al., 2013), and ESCRT-III, which cleaves the bud neck from its cytosolic face (Adell and Teis, 2011; Wollert et al., 2009).
We investigated the role of initial HIV-1 internalization in subsequent viral sequestration in the vesicular compartments of polarized tonsil, cervical and foreskin epithelial cells. Monostratified polarized epithelial cells may serve as a model for one of the sheets of stratified mucosal epithelium that are attached to one another by lateral junctions (McCaffrey and Macara, 2011; Muroyama and Lechler, 2012; St Johnston and Sanson, 2011; Tugizov et al., 2003, 2013b, 2011, 2012). Polarized epithelial cells have highly organized and functional vesicular/endosomal compartments (Rodriguez-Boulan and Macara, 2014) and are therefore suitable for modeling HIV-1 transmission via the mucosal epithelium. Our findings show that HIV endocytosis and macropinocytosis lead to sequestration of virions into late endosomes, including MVB and vacuoles. As we showed recently (Yasen et al., 2017), the interaction of activated lymphocytes with epithelia sequestering HIV-1 initiates the spread of virus from epithelial cells into lymphocytes. Thus, intravesicular HIV-1 sequestration may contribute to the molecular pathogenesis of viral spread from mucosal epithelial cells to virus-susceptible immune cells.
2. Results
2.1. HIV-1 internalization into epithelial cells is facilitated by multiple endocytic pathways
To identify the pathways of HIV-1 internalization into epithelial cells, we examined viral penetration in epithelial cells using pharmacologic inhibitors of clathrin- and caveolin/lipid raft-mediated endocytosis and macropinocytosis, which are major entry pathways of multiple viruses. Polarized tonsil epithelial cells were pretreated with increasing concentrations of chlorpromazine and nystatin, which are inhibitors of clathrin- and caveolin/lipid raft -mediated endocytosis, respectively (Ivanov, 2008a, b). Cells were also treated with amiloride, which inhibits macropinocytosis (Ivanov, 2008a; Saeed et al., 2010). These drugs are widely used for blocking endocytosis and macropinocytosis by many viruses, including HIV (Carter et al., 2011, 2009; Daecke et al., 2005; Dorosko and Connor, 2010; Ferreira et al., 2015; Grigorov et al., 2006; Kinlock et al., 2014; Liu et al., 2002; Mikulak et al., 2009; Miyauchi et al., 2009; Platt et al., 2014; Sloan et al., 2013). After 1 h of treatment, dual-tropic HIV-1SF33 internalization was examined from the apical (AP) surface of epithelium by p24 ELISA. Data showed that all three drugs reduced viral internalization in a dose-dependent manner (Fig. 1A). The highest concentrations of chlorpromazine (20 μM), nystatin (12 μg/ml) and amiloride (100 μM) were used to examine transepithelial resistance (TER) and the viability of polarized cells. None of the drugs reduced TER or viability (Fig. S1A and B, upper panels), i.e., drug treatment did not alter cell polarity. These concentrations of drugs were used in the rest of the study.
Fig. 1.
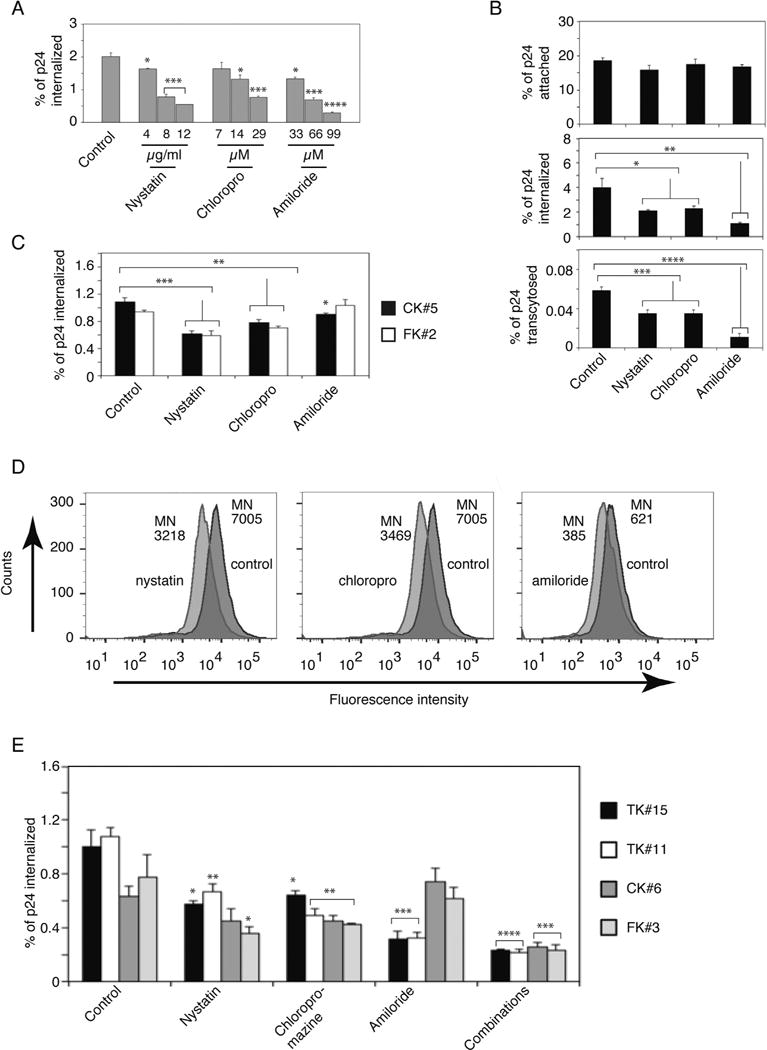
HIV-1 internalization into polarized tonsil, cervical and foreskin epithelial cells is facilitated by multiple pathways. (A) Polarized tonsil epithelial cells (TK#3) were pretreated with increasing concentrations of chlorpromazine, nystatin, or amiloride for 1 h. Dimethyl sulfoxide-treated cells served as a control. For virus internalization, HIV-1SF33 at 10 ng/insert of p24 was added to cells and incubated for 2 h at 37 °C. Cells were washed, trypsinized, and lysed, and intracellular virions were detected by p24 ELISA. Amount of internalized virions was expressed as a percentage of the initial viral inoculum. (B) Polarized tonsil TK#3 cells were pretreated with 12 μg/ml nystatin, 20 μM chlorpromazine or 100 μM amiloride for 1 h. For HIV-1SF33 attachment, cells were incubated with virus (10 ng/insert of p24) for 2 h at 4 °C, washed, detached and lysed. For virus internalization, virions were added to cells and incubated for 2 h at 37 °C. Viral transcytosis was measured from the lower chambers of Transwell inserts after 4 h of virus incubation from the AP surface of cells. HIV-1 attachment, internalization and transcytosis were measured by p24 ELISA. (C) Internalization of HIV-1SF33 was examined in polarized cervical CK#5 and foreskin FK#2 cells pretreated with nystatin, chlorpromazine or amiloride. (D) Tonsil TK#15 cells were pretreated with nystatin, chlorpromazine or amiloride or left untreated as control. After 1 h, nystatin- or chlorpromazine-treated cells were washed and incubated with 1 mg/ml of rhodamine-labeled dextran-10 for 1 h. Amiloride-treated cells were incubated with Texas Red-labeled dextran-70 for 1 h. Penetration of dextran was measured by flow cytometry. MN, mean of fluorescence intensity. (E) Tonsil TK#15 and TK#11, cervical CK#6 and foreskin FK#3 cells were pretreated with nystatin, chlorpromazine or amiloride, and their combinations, and were then used for internalization of HIV-1SF33. (A, B, D and E). Data are shown as mean ± SEM of three independent experiments (n=3). *P < 0.01, **P < 0.001, ***P < 0.0001 and **** P < 0.00001 compared with controls.
In the next experiments we examined the role of chlorpromazine, nystatin and amiloride in the attachment, internalization and transcytosis of HIV-1SF33 from the AP surface of epithelium. Virus attachment to the cell surface was measured at 4 °C, and intracellular and transcytosed virions were evaluated at 37 °C by p24 ELISA. Data showed that ~ 20% of initially inoculated virions were attached to the cell surface (Fig. 1B); none of the drugs significantly changed virus attachment to the AP surface (Fig. 1B, upper panel). Approximately 4% of initially added virions were internalized into cells, and all three drugs reduced viral internalization; inhibitors reduced clathrin- and caveolin/lipid raft-mediated HIV endocytosis by ~ 50%, and the inhibitor of macropinocytosis, amiloride, led to ~ 60% reduction of viral internalization (Fig. 1B, middle panel). HIV-1SF33 transcytosis was approximately ~0.06% of the initial inoculum, indicating that more than 95% of internalized virions were trapped in the cells. Nystatin or chlorpromazine reduced viral transcytosis by ~ 45%, and amiloride reduced it by ~ 80% (Fig. 1B, lower panel).
Analysis of the internalization of HIV-1SF33 in cervical and foreskin epithelial cells showed that virus internalization was significantly (~ 30–40%) reduced by inhibitors of clathrin- and caveolin/lipid raft-mediated endocytosis (Fig. 1C). However, the inhibitor of macropinocytosis, amiloride, had less of an effect on virus internalization in cervical cells (~ 15%). Amiloride did not reduce virus internalization into foreskin epithelial cells.
To verify the functional effect of drugs for endocytosis and macropinocytosis, tonsil TK#15 cells were treated or not treated with nystatin, chlorpromazine or amiloride. After 1 h, nystatin- or chlorpromazine-treated cells were washed and incubated with the endocytosis marker rhodamine-labeled dextran (10 kDa) (Li et al., 2015) (Fig. 1D). Amiloride-treated cells were incubated with Texas Red-labeled dextran (70 kD), which is a macropinocytosis marker (Commisso et al., 2014; Falcone et al., 2006; Li et al., 2015). Flow cytometry analysis showed that both nystatin and chlorpromazine reduced dextran-10 penetration by ~ 50%. Amiloride reduced the penetration of dextran-70 by ~ 45%.
The effect of drugs on HIV-1 internalization was validated in tonsil, cervical and foreskin epithelial cells derived from additional donors. These data showed that all three internalization pathways, i.e., clathrin-and caveolin/lipid raft-mediated endocytosis and macropinocytosis, are involved in HIV-1SF33 penetration into tonsil epithelial cells (Fig. 1E). However, macropinocytosis was not highly relevant for virus internalization in cervical and foreskin epithelial cells. A combination of all three drugs did not lead to complete inhibition of viral penetration in tonsil, cervical or foreskin cells, suggesting that HIV internalization may also occur by unidentified penetration pathway(s).
2.2. HSPG, GalCer and TIM-1 on the epithelial surface play a role in HIV internalization
To study the role of HIV-1 interaction with the epithelial surface in virus attachment, internalization and transcytosis, we preincubated cells with antibodies against HSPG and GalCer, which are well-known epithelial attachment molecules for HIV-1 (Bobardt et al., 2007; Bomsel and Alfsen, 2003; Fantini et al., 1997; Herrera et al., 2016; Tugizov et al., 2011). Since amiloride, the inhibitor of macropinocytosis, inhibits HIV-1 internalization (Fig. 1), we also preincubated cells with antibodies to TIM-1, which facilitates entry of various viruses by macropinocytosis. HSPG and GalCer are expressed on the AP surface of oral and genital mucosal epithelium and facilitate HIV-1 attachment and internalization in epithelial cells (Bobardt et al., 2007; Fantini et al., 1997; Herrera et al., 2016; Kumar et al., 2006; Tugizov et al., 2012). Western blot analysis of TIM-1 expression in tonsil epithelial cells showed that TIM-1 was detected in tonsil epithelium of all 12 independent donors (Fig. 2A). In the domain-specific surface biotinylation assay, TIM-1 expression was detected exclusively in AP membranes of polarized tonsil, cervical and foreskin epithelial cells (Fig. 2B).
Fig. 2.
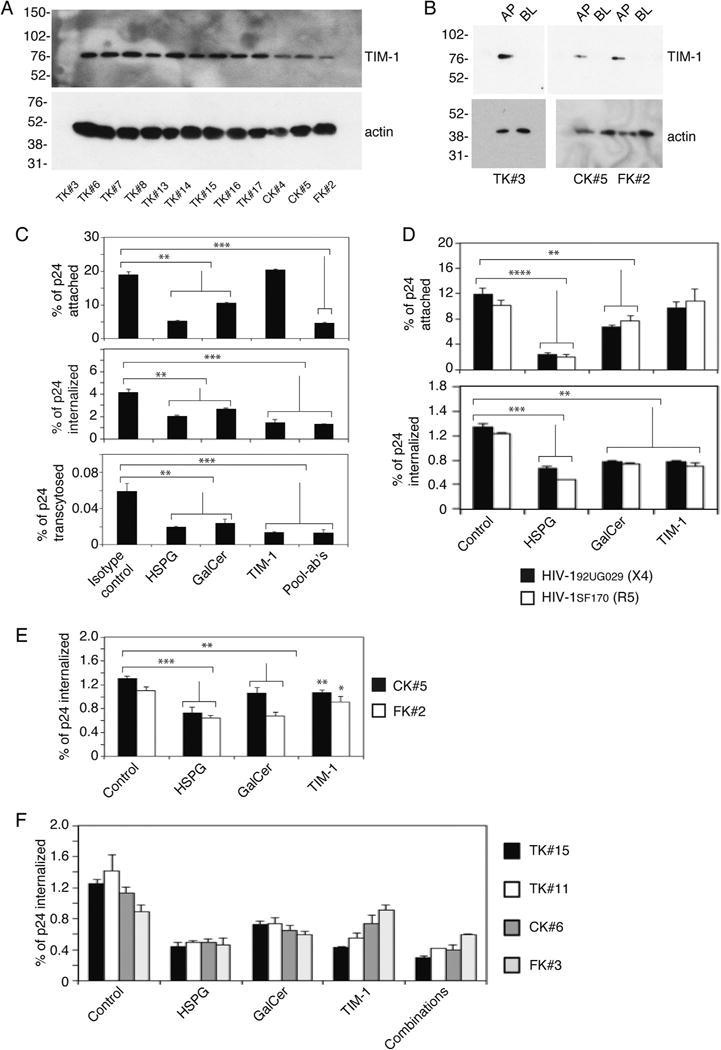
HSPG, GalCer and TIM-1 on the epithelial surface play a role in HIV internalization via endocytosis and macropinocytosis. (A and B) TIM-1 expression in tonsil, cervical and foreskin epithelial cells was detected by Western blot (A) and domain-specific biotinylation assays (B). (C) Tonsil cells TK#8 were pretreated with antibodies to GalCer, HSPG and TIM-1 or their combinations for 1 h. Cells treated with isotype antibodies served as a control. HIV-1SF33 attachment, internalization and transcytosis in antibody-treated and control cells were measured by p24 ELISA. (D) Polarized tonsil TK#8 cells were pretreated with antibodies to GalCer, HSPG and TIM-1, and attachment and internalization of X4- and R5-tropic strains of HIV-1 were examined. (E) Cervical CK#5 and foreskin FK#2 cells were incubated with antibodies to GalCer, HSPG and TIM-1, and HIV-1SF33 internalization was examined. (F) Polarized tonsil TK#15 and TK#11, cervical CK#6, and foreskin FK#3 were treated with antibodies to GalCer, HSPG and TIM-1 and their combinations. These cells then were used for HIV-1SF33 internalization. (A, D, E and F). Data are shown as mean ± SEM of three independent experiments (n=3). *P < 0.01, **P < 0.001, ***P < 0.0001 and **** P < 0.00001 compared with controls.
Analysis of HIV-1SF33 binding to tonsil epithelial cells showed that antibodies against HSPG and GalCer significantly reduced virus binding to the AP surface of polarized epithelial cells (Fig. 2C, upper panel). In contrast, anti-TIM-1 antibodies did not affect virus binding. The pool of antibodies to HSPG, GalCer and TIM-1 did not lead to complete inhibition of HIV-1 attachment. Examination of HIV-1SF33 internalization and transcytosis showed that all three antibodies to HSPG, GalCer and TIM-1 significantly reduced viral internalization and transcytosis (Fig. 2C, middle and lower panels, respectively). However, complete inhibition of virus internalization and transcytosis was not detected by a combination of these antibodies. Inhibition of attachment and internalization of HIV-1 by anti-HSPG and anti-GalCer antibodies showed that both antibodies reduced attachment and internalization of X4- and R5-tropic viruses, similar to the inhibition of dual-tropic virus internalization and attachment (Fig. 2D). TIM-1 antibodies did not lead to significant reduction of attachment of these viruses to the tonsil epithelial cells.
Analysis of internalization of HIV-1SF33 in cervical and foreskin epithelial cells by antibodies showed that virus internalization was significantly (~ 30–40%) reduced by anti-HSPG and anti-GalCer antibodies (Fig. 2E). However, the reduction of virus internalization by TIM-1 antibodies and amiloride was less significant (~ 7–15%). These effects were validated in tonsil, cervical and foreskin epithelial cells isolated from additional donors (Fig. 2F), which showed similar data to that in Fig. 2C, D and E.
In parallel experiments we examined TER and cell viability of antibody-treated and untreated (control) polarized tonsil epithelial cells. Data showed that antibody treatment did not reduce TER (Fig. S1A, lower panel) and did not show a toxic effect on cells (Fig. S1B, lower panels).
These findings showed that HIV-1 internalization into tonsil epithelial cells may occur by multiple pathways, including clathrin- and caveolin/lipid raft-mediated endocytosis and macropinocytosis, which may be facilitated by HSPG, GalCer and TIM-1. Virus internalization in cervical and foreskin epithelial cells mainly occurs by clathrin- and caveolin/lipid raft-mediated endocytosis. The role of TIM-1-associated macropinocytosis in HIV-1 internalization in cervical and foreskin epithelial cells is less relevant.
2.3. TIM-1 of tonsil epithelial cells promotes HIV-1 internalization by macropinocytosis
To better understand TIM-1-associated HIV-1 macropinocytosis into tonsil epithelial cells, we examined HIV-1SF33 internalization in amiloride-pretreated tonsil cells from 9 independent donors. Data showed that amiloride significantly reduced virus internalization in 7 of 9 (75%) donor cell lines (Fig. 3A). Amiloride treatment of tonsil epithelial cells from 2 donors did not reduce HIV internalization despite our confirmation of apical TIM-1 expression (Fig. 3B).
Fig. 3.
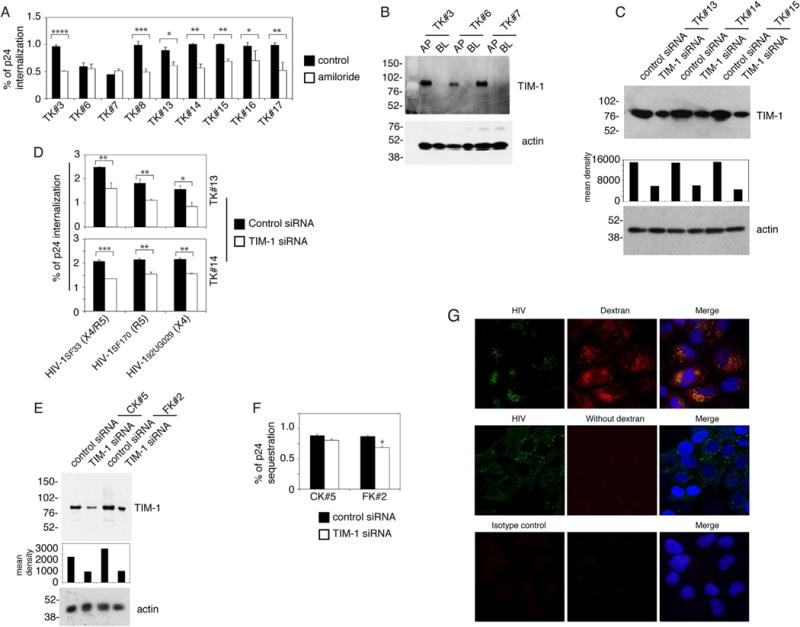
TIM-1-associated HIV-1 macropinocytosis in tonsil epithelial cells. (A) Polarized tonsil epithelial cells from 9 independent donors were pretreated with amiloride and then HIV-1SF33 internalization was examined. Data are shown as mean ± SEM (bars) of triplicate values. *P < 0.01, **P < 0.001, ***P < 0.0001 and ****P < 0.00001. (B) Cell surface expression of TIM-1 was detected in 3 polarized tonsil epithelial cells by domain-specific surface biotinylation. (C) Tonsil epithelial cells TK#13, TK#14 and TK#15 were transfected with TIM-1 siRNA and control siRNA. After 3 days, TIM-1 expression was examined by Western blot. The mean density of TIM-1 protein bands is shown under the blot. β-Actin was detected, confirming equal loading. (D) Dual-tropic HIV-1SF33, R5-tropic HIV-1SF170 and X4-tropic HIV-192UG029 viruses were added to the apical surface of tonsil epithelial cells transfected with TIM-1 siRNA or control siRNA. After 2 h, cells were trypsinized and virus internalization was examined by ELISA p24. (E) Cervical CK#5 and foreskin FK#2 cells were transfected with TIM-1 siRNA and control siRNA, and 3 days later TIM-1 expression was detected by Western blot. The density of protein bands was measured; the mean density is shown under the blot. β-Actin was examined in cell lysates and used as a loading control. (F) Cervical and foreskin epithelial cells were transfected with TIM-1 siRNA, and control siRNA was used for HIV-1SF33 internalization. (G) HIV-1SF33 and Texas Red-labeled dextran-70 were added simultaneously to the AP surface of polarized tonsil epithelial cells (TK#3) for 30 min at 37 °C, and cells were fixed and immunostained for HIV-1 p24 (green). Similar data were obtained in three independent experiments. (D and F) Data represent one of two independent experiments and are shown as mean ± SEM of triplicate values. *P < 0.01, **P < 0.001 and ***P < 0.0001.
To further examine the role of TIM-1 in HIV-1 macropinocytosis, TIM-1 expression in 3 tonsil cells was inhibited by TIM-1-specific siRNA transfection (Fig. 3C), which reduced TIM-1 expression by 60–70% compared to control siRNA (Fig. 3C). Analysis of dual-tropic HIV-1SF33 and, X4- and R5-tropic strains of HIV-1 in tonsil cells transfected with TIM-1 siRNA showed that viral internalization was reduced by ~ 35–45% (Fig. 3D).
To study the role of TIM-1 in HIV-1 macropinocytosis in cervical and foreskin epithelial cells, we inhibited TIM-1 expression by siRNA transfection (Fig. 3E) and then examined HIV-1SF33 internalization (Fig. 3F). Inhibition of expression of TIM-1 by siRNA in cervical epithelial cells did not show a reduction in HIV-1 internalization (Fig. 3F). The lower expression of TIM-1 was associated with a slight reduction (~ 15%) in HIV-1SF33 internalization into foreskin epithelial cells.
To confirm HIV-1 penetration by macropinocytosis, we added the HIV-1 and macropinocytosis marker Texas Red-labeled dextran-70 (Commisso et al., 2014; Falcone et al., 2006; Li et al., 2015) to AP membranes of polarized tonsil epithelial cells for 30 min, and cells were then analyzed by fluorescence microscopy. Analysis showed that HIV-1 was colocalized with dextran-70 (Fig. 3G), consistent with virus internalization by macropinocytosis.
2.4. HIV-1 sequestration in epithelial cells initiated by viral interaction with HSPG, GalCer and TIM-1 of the epithelial surface, and virus internalization via endocytosis and macropinocytosis
In our recent work, we have shown that HIV-1 may sequester in the vesicular compartments of epithelial cells without release, i.e., virions are trapped in the endosomes, including MVBs and vacuoles (Yasen et al., 2017). To determine if HIV-1 interaction with the epithelial surface is able to initiate the subsequent sequestration of virus in the vesicles, we studied the role of HSPG, GalCer and TIM-1 in HIV-1 sequestration in tonsil epithelial cells. Cells were preincubated with antibodies against HSPG, GalCer and TIM-1 or a pool of their isotype controls. The dual-tropic HIV-1SF33 and X4- and R5-tropic isolates of HIV-1 were added to the AP surface of polarized cells. Analysis of viral sequestration after 6 days showed that all 3 antibodies reduced sequestration of 3 strains of HIV-1 by ~ 40–60% (Fig. 4A).
Fig. 4.
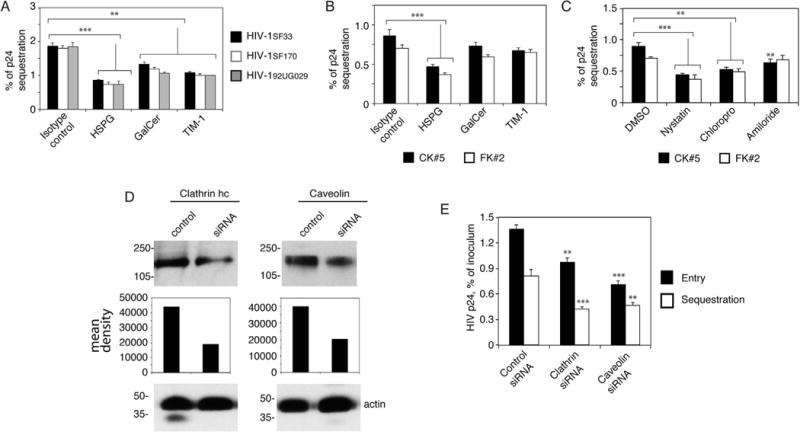
HIV-1 endocytosis and macropinocytosis in tonsil epithelial cells and virus endocytosis in cervical and foreskin epithelial cells play a role in the sequestration of virions in the vesicles. (A) Polarized tonsil epithelial cells were pretreated with antibodies to HSPG, GalCer and TIM-1 for 1 h. Cells treated with isotype antibodies served as a control. Cells were washed and incubated with HIV-1SF33, HIV-1SF170 and HIV-192UG029 viruses for 2 h, washed again, and cultured for 6 days. Cells were collected by trypsinization, and intracellular virions were examined by p24 ELISA. (B) Polarized cervical and foreskin epithelial cells were preincubated with antibodies to HSPG, GalCer and TIM-1. Cells were incubated with HIV-1SF33 and after 6 days were examined for intracellular virus. (C) Cervical and foreskin epithelial cells were treated with chlorpromazine, nystatin, or amiloride for 1 h. Cells were incubated with HIV-1SF33 for 2 h, washed, and after 6 days were examined for intracellular virus. (A-C) Data represent one of two independent experiments and are shown as mean ± SEM of triplicate values. **P < 0.001 and ***P < 0.0001 compared with controls. (D) Tonsil epithelial cells were transfected with clathrin or caveolin siRNA, or control siRNA. After 3 days, clathrin or caveolin expression was examined by Western blot. The mean density of protein bands is shown under the blot. β-Actin was detected in the samples, confirming equal loading. (E) HIV internalization after 2 h and viral sequestration after 3 days were examined in tonsil epithelial cells transfected with clathrin or caveolin siRNA, or control siRNA. Data represent one of two independent experiments and are shown as mean ± SEM of triplicate values. **P < 0.007 and ***P < 0.001 compared with controls.
We also examined the role of HSPG, GalCer and TIM-1 (Fig. 4B), and clathrin- and caveolin/lipid raft-mediated endocytosis and macropinocytosis (Fig. 4C) in HIV-1SF33 sequestration in cervical and foreskin epithelial cells. The data showed that inhibition of HIV-1SF33 interaction with HSPG reduced subsequent viral sequestration by ~ 50% (Fig. 4B). HIV-1SF33 interaction with GalCer and TIM-1 reduced viral sequestration by ~ 7–15%. Inhibitors of clathrin- and caveolin/lipid raft-mediated endocytosis of HIV-1SF33 in cervical and foreskin cells reduced viral sequestration by ~ 50–60% (Fig. 4C). Inhibition of macropinocytosis reduced HIV-1SF33 sequestration by 20% in cervical epithelial cells and 5% in foreskin epithelial cells.
To confirm the role of clathrin- and caveolin/lipid raft-mediated HIV internalization in viral sequestration, we inhibited expression of clathrin and caveolin by transfection of tonsil epithelial cells with siRNAs against clathrin heavy chain and caveolin-1, respectively. Western blotting of transfected cells after 3 days showed that siRNA reduced clathrin and caveolin expression by ~ 60% and ~ 50%, respectively (Fig. 4D). Then, we examined HIV-1SF33 internalization after 2 h and sequestration after 3 days. Data showed that silencing clathrin gene expression reduced viral internalization and sequestration by ~ 35% and ~ 50%, respectively (Fig. 4E). Inhibition of caveolin expression leads to reduction of HIV internalization and sequestration by ~ 60% and ~ 45%, respectively.
Together, these data suggest that the initial internalization of HIV-1 by clathrin- and caveolin/lipid raft-mediated endocytosis and TIM-1-associated macropinocytosis could be relevant for subsequent viral sequestration in the vesicles.
We have shown that HIV-1 sequestration mostly occurs in MVB and vacuoles of epithelial cells (Yasen et al., 2017). To identify the role of initial internalization pathways of virus in its subsequent sequestration in MVB and vacuoles, we silenced expression of the critical proteins of MVB—the hepatocyte growth factor-regulated tyrosine kinase substrate (Hrs)—and of vacuoles—rabankyrin—by siRNA transfection, respectively. Western blotting showed that siRNA-mediated inhibition of Hrs and rabankyrin was ~ 70–90% (Fig. 5A). Then, cells were pretreated with nystatin, chlorpromazine or amiloride. Analysis of HIV-1SF33 sequestration in tonsil cells showed that virus sequestration in Hrs-siRNA-transfected cells was reduced by nystatin (~ 27%) and amiloride (~ 35%), indicating that inhibition of initial caveolin/lipid raft-mediated endocytosis and macropinocytosis of virus also led to the subsequent reduction of viral sequestration in cells lacking Hrs expression (Fig. 5B, upper panel). However, chlorpromazine did not change viral sequestration in these cells. Inhibition of all 3 pathways of viral internalization reduced subsequent virus sequestration (~ 40–65%) in cells lacking rabankyrin expression, suggesting that clathrin- and caveolin/lipid raft-mediated endocytosis and macropinocytosis may lead to HIV-1 sequestration into vacuoles (Fig. 5B, lower panel). A similar trend of reduction of viral sequestration was observed in Hrs- and rabankyrin-siRNA-transfected cervical and foreskin epithelial cells by nystatin and chlorpromazine (Fig. 5C). However, amiloride did not reduce HIV-1 sequestration in either Hrs- and rabankyrin-siRNA-transfected cervical cells or Hrs-siRNA-transfected foreskin cells. Amiloride reduced viral sequestration in rabankyrin-siRNA-transfected foreskin cells by ~ 25%. These data suggest that after HIV internalization by clathrin- and caveolin/lipid raft, the Hrs and rabankyrin may play an important role in the regulation of HIV sequestration in MVB and vacuoles, respectively.
Fig. 5.
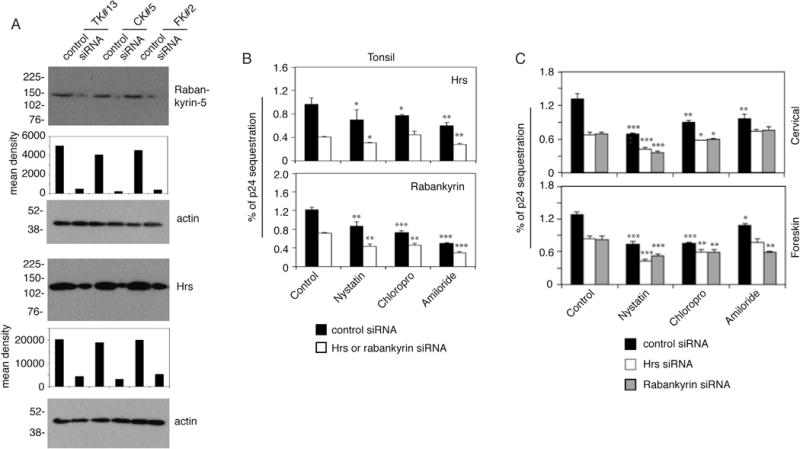
Initial internalization pathways of HIV-1 determine subsequent sequestration of virus in MVB and vacuoles. (A) Tonsil, cervical and foreskin epithelial cells were transfected with Hrs or rabankyrin siRNA, or control siRNA. After 3 days, Hrs and rabankyrin expression was examined by Western blot. The mean density of protein bands is shown under the blot. β-Actin was detected in the cell lysates, confirming equal loading. (B and C) Tonsil (B), cervical and foreskin (C) epithelial cells were transfected with Hrs or rabankyrin siRNA, or control siRNA. After 3 days, cells were treated with chlorpromazine, nystatin, or amiloride for 1 h. HIV-1SF33 was added to the apical surface for 2 h, washed, and after 3 days intracellular virus was examined. (B and C) Data represent one of two independent experiments and are shown as mean ± SEM of triplicate values. *P < 0.01, **P < 0.001 and ***P < 0.0001 compared with controls.
2.5. Inhibition of MVB formation reduced HIV-1 sequestration, and acidification of vesicles increased viral sequestration
For confirmation of the role of MVB in HIV-1 sequestration HIV-1, we pretreated polarized tonsil epithelial cells with LY294002 (10 μM), an inhibitor of phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) that is critical for MVB formation (Fernandez-Borja et al., 1999). In parallel experiments, we pretreated cells with GW4869 (10 μM), a sphingomyelinase inhibitor, which inhibits ceramide-mediated formation of MVB (Wang et al., 2014). One set of cells were treated with a combination of LY294002 and GW4869 (5 μM each). After 24 h, HIV-1SF33 was added to the AP surface for viral sequestration for 2 days in the presence of LY294002 and GW4869 at lower concentrations (1 μM each). Analysis of intracellular HIV-1 showed that both treatments reduced intraepithelial viral sequestration by ~ 60% (Fig. 6A). The decrease in HIV-1 sequestration was higher (~ 70%) with the combined drugs than with the individual treatments. Together, data from siRNAs (Fig. 5B and C) and inhibitors (Fig. 6A) indicated that the biogenesis of MVB and macropinosomes/vacuoles is important for HIV-1 in-traepithelial sequestration.
Fig. 6.
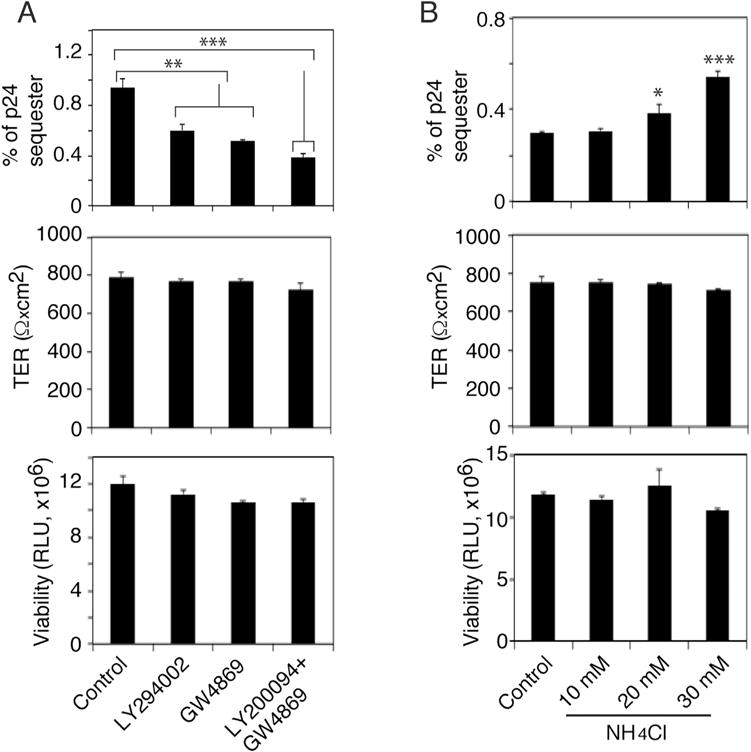
HIV-1 sequestration in MVB and macropinosomes/vacuoles. (A) Polarized tonsil epithelial cells were treated with LY294002 and/or GW4869; after 24 h, HIV-1SF33 was added to the AP surface for 2 days in the presence of the drugs. Cells were examined for intracellular virions (upper pane), TER (middle panel) and cell viability (lower panel). (B) Polarized tonsil cells were treated with various concentrations of NH4Cl for 1 h and then exposed to HIV-1SF33. Cells were cultured for 48 h in the presence of NH4Cl and examined for intracellular virus (upper pane), TER (middle panel) and viability (lower panel). Data represent one of two independent experiments and are shown as mean ± SEM of triplicate values. *P < 0.01, **P < 0.001 and ***P < 0.0001 compared with controls.
We have shown that HIV-1 sequestration is gradually reduced at later time points (Yasen et al., 2017). This suggests the possible fusion of vesicles containing HIV-1, including MVB and vacuoles, with highly acidic lysosomes, which may inactivate the virus. To test this hypothesis, we pretreated polarized tonsil epithelial cells with various concentrations of ammonium chloride (NH4Cl), which inhibits acidification of vesicular pH (Fredericksen et al., 2002). After 2 h, cells were exposed to HIV-1SF33 and cultured with NH4Cl for 2 days. Intracellular virions were then examined by ELISA p24, which showed that NH4Cl treatment increased intracellular HIV-1 in a dose-dependent manner, unlike untreated control cells (Fig. 6B). These results indicate that a gradual reduction of sequestered HIV-1 concentrations may be due to fusion of MVB/vacuoles containing HIV-1 with lysosomes.
Analysis of TER and cell viability of polarized cells treated with LY294002, GW4869 and NH4Cl, as described in the above experiments, showed that none of the drugs showed a significant toxic effect on the cells or reduced their TER (Fig. 6A and B, middle and lower panels), indicating that cell polarity was intact.
2.6. HIV sequestration is not associated with membrane invagination
We have shown that HIV-1 is sequestered in endosomes of polarized mucosal epithelial cells (Yasen et al., 2017). Macrophages and dendritic cells may sequester HIV-1 in the surface-accessible intracellular compartments and within the extracellular membrane invaginations (Cavrois et al., 2007; Yu et al., 2008). To confirm the intracellular nature of HIV-1 sequestration in mucosal epithelial cells, we first examined if we could remove sequestered virions by pronase, which removes extracellular virions (Cavrois et al., 2007). Polarized tonsil epithelial cells were incubated with HIV-1SF33 for 2 h at 4 °C to determine viral attachment or for 6 days at 37 °C to determine virus sequestration. Cells with attached or sequestered HIV-1 were treated with pronase. P24 ELISA showed that pronase treatment removed attached virions from the cell surface (Fig. 7A, upper panel). However, on day 6 of viral sequestration, pronase did not reduce viral concentration, indicating the absence of virions on the cell surface (Fig. 7A, lower panel). These data show that the sequestered virions were resistant to enzyme, indicating that virions are localized in the vesicular compartments.
Fig. 7.
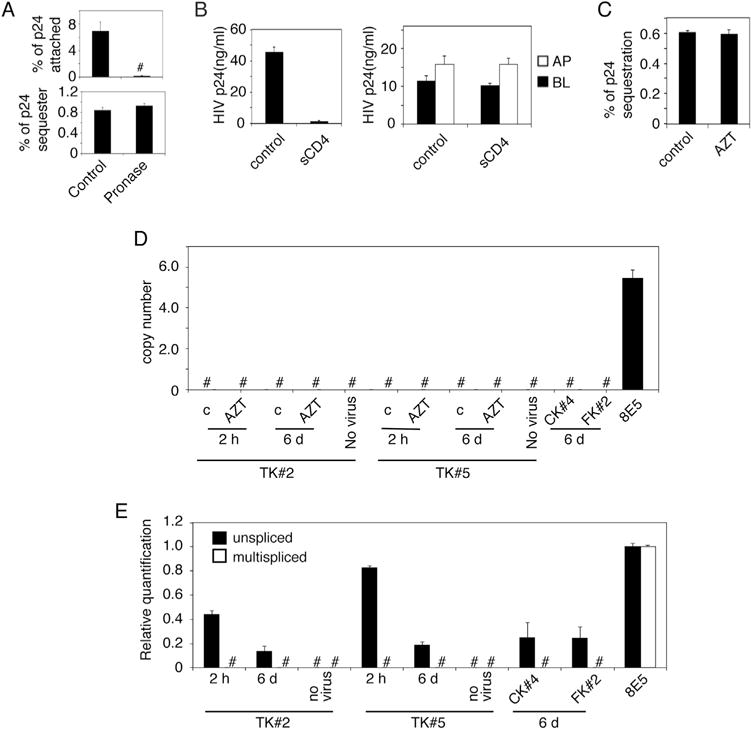
HIV-1 sequestration in polarized epithelial cells is not associated with membrane invagination and productive viral infection. (A) HIV-1SF33 was added to the AP surface of polarized tonsil epithelial cells for 1 h at 4 °C to determine viral attachment (upper panel). Another set of cells was used to determine sequestration of HIV-1SF33 for 6 days (lower panel). Cells with attached HIV-1 or sequestered HIV-1 were washed and treated with pronase. Untreated cells served as a control. Cells were collected and used for detection of attached and sequestered virions using ELISA p24. (B) HIV-1SF33 was added to the AP surface of tonsil cells and incubated at 4 °C for 1 h to allow virus attachment. sCD4 was added for 1 h at 4 °C (left panel). In parallel experiments, tonsil cells containing intracellular HIV for 6 days were exposed to sCD4 from AP and BL membranes for 1 h at 4 °C (right panel). Cells without sCD4 served as a control. Cells were washed and cocultivated with activated PBMC from AP membranes in cells with attached HIV and from AP or BL membranes in cells with sequestered virions for 4 h at 37 °C. PBMC were collected and cultured for 7 days and HIV infection was examined by ELISA p24. Data represent one of four independent experiments and are shown as mean ± SEM of triplicate values. (C) Polarized tonsil cells were pretreated with 10 μM AZT for 24 h, and HIV-1SF33 was added to the AP surface of cells for 4 h. Cells were washed, uninternalized virus was removed, and cells were cultured for 6 days with AZT. One set of cells with virus were untreated and served as a control. At day 6 cells were collected and examined for intracellular virus by ELISA p24. (D) Polarized tonsil TK#2 and TK#5 cells were treated with AZT for 24 h and exposed to HIV-1SF33. One set of cells were cultured for 2 h, and one set were cultured for 6 days. Polarized cervical CK#4 and foreskin FK#2 cells were exposed to HIV-1 and cultured for 6 days. As a positive control, we used an HIV-1-infected 8E5 T lymphocytic cell line. Cells were collected by trypsinization, and integration of HIV-1 DNA was examined using qPCR. (E) Polarized TK#2, TK#5, CK#4 and FK#2 cells were exposed to HIV-1SF33, and at 2 h or 6 days later, cells were collected and used for detection of unspliced and multispliced HIV-1 RNA by qPCR. Data represent one of three independent experiments and are shown as mean ± SEM of triplicate values.
We also confirmed HIV sequestration in the vesicular compartments by using soluble sCD4, which neutralizes extracellular virions in the membrane and in the surface-accessible intracellular compartments, but not in the intracellular vesicles (Cavrois et al., 2007). As a control, polarized cells were incubated with HIV-1SF33 for 1 h at 4 °C and then incubated with sCD4 for 1 h at 4 °C (Fig. 7B, left panel). In parallel experiments, cells with sequestered virions for 6 days were exposed to sCD4 from AP and BL surfaces and were incubated at 4 °C for 1 h (Fig. 7B, right panel). Cells were washed, and activated PBMC were added to the AP surface of cells with viral attachment and to the AP and BL surfaces of cells containing sequestered virions. In vivo, with inflammation, the interaction of HIV-susceptible cells with epithelial cells may occur from both AP and BL surfaces of mucosal epithelia. Thus, these experiments may model HIV spread from epithelia into lymphocytes, as shown in our recent work (Yasen et al., 2017). After 4 h PBMC were collected and grown for 7 days. HIV infectivity was examined by ELISA p24, which showed that sCD4 neutralized virus in attachment experiments, i.e., HIV-1 spread was inhibited by ~ 95–98%. In contrast, in sequestration experiments sCD4 did not inhibit HIV spread from epithelia to lymphocytes, confirming sequestration of virions in the intracellular vesicles/endosomes, which are not surface accessible.
2.7. HIV-1 sequestration in epithelial cells is not associated with productive viral infection
We examined the possible role of HIV-1 infection in tonsil epithelial cells with viral sequestration by using azidothymidine (AZT), which inhibits viral replication. Polarized tonsil epithelial cells were treated with 10 μM AZT one day and exposed to HIV-1SF33 the next. Untreated cells served as a control. Cells were cultured for 6 days, during which medium was refreshed with AZT. Analysis of intracellular virions in AZT-treated and untreated cells incubated for 6 days showed that a decrease in HIV-1 sequestration was not detected by AZT, indicating that viral sequestration is not due to viral replication (Fig. 7C).
To confirm the lack of HIV-1 replication in tonsil epithelial cells, we examined integrated HIV-1 DNA and nonspliced and multispliced viral RNA in polarized tonsil cells (TK#2 and TK#5) with HIV-1 sequestration by using real-time quantitative PCR (qPCR) (Fig. 7D and E). The CD4+ T lymphocyte line 8E5, chronically infected with HIV-1, served as a positive control (Serpente et al., 1993). To determine integration of HIV-1 DNA, we pretreated one set of cells with AZT and left another set untreated. Untreated and AZT-treated cells were exposed to HIV-1, and after 2 h or 6 days of incubation, cells were trypsinized and integrated HIV-1 DNA was determined. HIV-1-infected 8E5 lymphocytes were not treated with AZT, as they already contain integrated HIV-1 DNA (Vandegraaff et al., 2001). Tonsil epithelial cells with or without AZT treatment did not contain integrated HIV-1 DNA, in contrast to the positive control cells (Fig. 7D). Integration of HIV-1 DNA was also not detected in cervical and foreskin epithelial cells.
Next, we examined nonspliced and multispliced viral RNA in po-larized tonsil cells (TK#2 and TK#5) with HIV-1 sequestration after 2 h or 6 days by real-time qPCR. Both tonsil epithelial cell lines contain nonspliced HIV-1 RNA but not multispliced HIV-1 RNA (Fig. 7E). Similar data were obtained from cervical and foreskin cell lines. Thus, HIV-1 in polarized tonsil, cervical and foreskin epithelial cells did not replicate, confirming that intraepithelial viral sequestration is due to entry of virions into vesicular/endosomal compartments and not the result of productive HIV-1 infection.
3. Discussion
Recently, we showed that HIV-1 penetrates polarized tonsil, cervical and foreskin epithelial cells and is sequestered in the vesicles (Yasen et al., 2017). The interaction of activated CD4+ T lymphocytes with epithelial cells induces the spread of sequestered intraepithelial virions into lymphocytes, which may play a critical role in initial HIV-1 transmucosal transmission. In the present study, we have shown that the interaction of HIV-1 with epithelial surface proteins HSPG, GalCer and TIM-1, and internalization of virus by clathrin- and caveolin/lipid raft-associated endocytosis and macropinocytosis, leads to viral sequestration in the vesicles (Fig. 8).
Fig. 8.
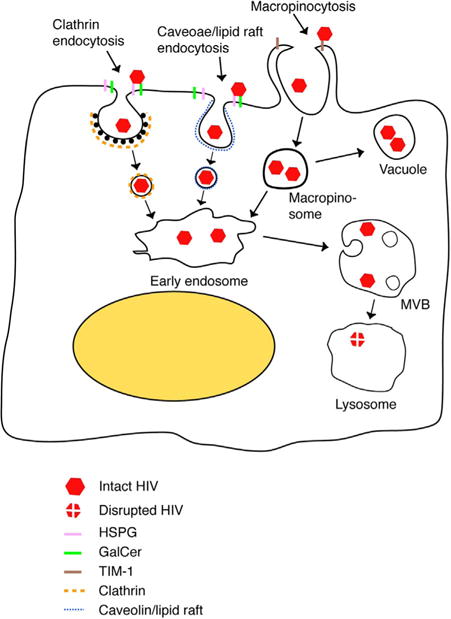
Model of HIV sequestration in mucosal epithelial cells. HIV-1 binding to HSPG and GalCer on the epithelial surface may initiate endocytosis of virions via clathrin- and caveolin/lipid raft – associated endocytosis, respectively. HIV interaction with TIM-1 may induce macropinocytosis of virions. HIV endocytosis and macropinocytosis deliver virions into the early endosomal compartment. Some virus containing vesicles and macropinosomes may not fuse with early endosomes and exist as independent vacuoles, establishing sequestration of virions. Maturation of early endosomes containing virions into MVBs leads to sequestration of virions in these endosomes. Fusion of MVBs and vacuoles containing HIV with lysosomes may lead to degradation of virus. However, lack of fusion of MVBs and vacuoles with lysosomes may generate intraepithelial viral reservoirs. HIV sequestration in MVBs and vacuoles of mucosal epithelial cells may play an important role in HIV transmission and development of HIV/AIDS.
It is well documented that HIV gp120 binds to HSPG through the V3 extracellular domain of gp120 (Batinic and Robey, 1992; Connell and Lortat-Jacob, 2013; Harrop and Rider, 1998; Rider et al., 1994; Roderiquez et al., 1995; Vives et al., 2005), leading to viral endocytosis and transcytosis in genital epithelial cells (Bobardt et al., 2007; Bomsel, 1997; Kinlock et al., 2014). We have shown that HIV-1 gp120 binding to HSPG mediates virus endocytosis in infant tonsil epithelial cells (Herrera et al., 2016). HSPG interaction with many viruses, including retroviruses and papillomaviruses, facilitates viral entry by using clathrin- and caveolin/lipid raft-dependent and independent pathways and macropinocytosis (Bobardt et al., 2007; Christianson and Belting, 2014; Connell and Lortat-Jacob, 2013; Grassel et al., 2016; Jones et al., 2005; Letian and Tianyu, 2010; Payne et al., 2007; Schelhaas et al., 2012). Human papillomavirus (HPV) types 16, 18 and 31 interaction with syndecan-1/HSPG and tetraspanin CD63 facilitate delivery of virus into MVB (Grassel et al., 2016). Post-endocytic interaction of the HPV/HSPG/CD63 complex with ESCRT protein Alix is critical for delivery of internalized virions to MVB (Grassel et al., 2016). HIV-1 interaction with HSPG is important for viral attachment, internalization and sequestration in tonsil, cervical and foreskin epithelial cells, suggesting that HSPG may play a major role in virus sequestration in oral and genital mucosal epithelium.
HIV gp120 V3 interaction with the glycolipid GalCer is critical for GalCer- mediated viral internalization (Campbell et al., 2001; Chazal and Gerlier, 2003; Ewers and Helenius, 2011; Fantini et al., 1997), which could be associated with caveolin/lipid raft-mediated virus endocytosis. Inhibition of HIV-1 internalization and sequestration by nystatin from the AP surface of polarized cells suggests that caveolin/lipid raft-associated endocytosis may play a role in delivery of virus to MVB. Lipid raft membrane domains have been detected in purified MVB, including their limiting membranes and intraluminal vesicles, suggesting that GalCer-associated HIV-1 endocytosis may lead to delivery of virions into MVB (Sobo et al., 2007). Nystatin also inhibits caveolae-associated endocytosis; however, in polarized epithelial cells, including tonsil epithelia, caveolae/caveosomes are localized to basolateral membranes (Vogel et al., 1998) and caveolae-mediated endocytosis occurs mostly from basolateral membranes (Tugizov et al., 2013b).
Accumulating evidence indicates that TIM-1 and TIM-1 family proteins facilitate the entry of multiple viruses, including arenavirus, alphavirus, filovirus and flavivirus, through envelope-associated PS (Jemielity et al., 2013; Moller-Tank et al., 2013). Surface exposure of PS in the viral envelope mimics virions as apoptotic bodies, and TIM-1 binding to virion-associated PS initiates viral entry by macropinocytosis (Jemielity et al., 2013; Moller-Tank et al., 2013), which resembles engulfing of apoptotic cells by macrophages (Kobayashi et al., 2007). In activated CD4+ T lymphocytes, TIM-1 promotes HIV-1 entry; however, upon viral egress, TIM-1 binding to virion-associated PS retains HIV-1 on the cell surface (Li et al., 2014). We found that, in polarized tonsil, cervical and foreskin epithelial cells, TIM-1 expression is restricted only on the apical surface. However, TIM-1-facilitated HIV internalization by macropinocytosis was detected mostly in tonsil epithelial cells. TIM-1-associated virus internalization could be less relevant in cervical and foreskin epithelial cells than in tonsil cells. Such cell type-specific function of TIM-1 in HIV-1 macropinocytosis could be due to the differential expression of partner and/or signaling protein(s) for TIM-1, which may be abundantly expressed in tonsil epithelium but not in cervical and foreskin epithelia. HIV-1 release from BL of tonsil epithelial cells due to viral transcytosis was not affected (Yasen et al., 2017). Normal BL HIV release could be due to a lack of TIM-1 in BL membranes of epithelial cells; i.e., released virions were not retained by TIM-1 in the BL membranes. TIM-1 was detected in early and late endosomes (Echbarthi et al., 2015), suggesting that TIM-1-induced HIV-1 macropinocytosis may lead to viral sequestration in MVB and vacuoles. The level of PS enrichment on the virion envelope may determine TIM-1-mediated viral macropinocytosis and the virus’s subsequent sequestration into vesicles. The association of PS with the virus envelope depends on multiple factors, including the lipid composition of virus-replicating CD4+ T lymphocytes, macrophages, and dendritic/Langerhans cell membranes (Aloia et al., 1993a; Callahan et al., 2003; Gu et al., 2017), i.e., the origin of the virus may also play a role in viral macropinocytosis and sequestration.
HIV-1 has been detected in the endosomes, MVB, and vacuoles of epithelial cells (Daecke et al., 2005; Dorosko and Connor, 2010; Ferreira et al., 2015; Grigorov et al., 2006; Kinlock et al., 2014; Mikulak et al., 2009; Orenstein, 2007; Tugizov et al., 2011; Yasen et al., 2017). However, critical extra- and intraepithelial molecules regulating vesicular trafficking of virions are still poorly investigated. Here we showed that initial HIV-1 interaction with mucosal epithelia occurs through HSPG, GalCer and TIM-1, which may trigger viral internalization by clathrin- and caveolin/lipid raft-associated endocytosis and macropinocytosis. HIV-1 internalized by endocytosis and macropinocytosis may reach the early endosomes, which mature into MVB. Macropinosomes may also fuse with each other to form large vacuoles, which may exist independently from the early and late endosomes (Falcone et al., 2006; Hewlett et al., 1994; Racoosin and Swanson, 1993). Such vacuoles may also be formed by clathrin-coated endocytic vesicles (Araki et al., 2006; Hamasaki et al., 2004; Sachse et al., 2002; Schnatwinkel et al., 2004). Reduction of virus sequestration by antibody-mediated inhibition of viral interaction with GalCer, HSPG and TIM-1, and by drug-mediated inhibition of viral endocytosis and macropinocytosis, indicates that HIV-1 penetration into early endosomes and macropinosomes determines the subsequent viral sequestration in MVB and vacuoles.
The lack of difference in penetration and sequestration of dual-tropic and R5- and X4-tropic HIV-1 in epithelial cells could be due to initial viral interaction with GalCer, HSPG and/or TIM-1, which do not play a role in selective entry of R5- or X4-tropic viruses (Baleux et al., 2009). Oral and genital mucosal epithelia may express HIV-1 coreceptors CCR5 and CXCR4; however, they lack expression of CD4 (Bobardt et al., 2007; Bomsel, 1997; Herrera et al., 2016; Hocini et al., 2001; Hocini and Bomsel, 1999; Saidi et al., 2007; Tugizov et al., 2011). CD4 plays a critical role in the interaction of viral glycoproteins gp120/41 with CCR5 and/or CXCR4, which determine selective viral tropism in lymphocytes, macrophages and dendritic cells (Wilen et al., 2012). However, the lack of CD4 expression in epithelial cells suggests that mucosal epithelia may not play a specific role in the selective sequestration/transmission of R5-tropic over X4-tropic HIV-1.
An increase in HIV-1 sequestration by NH4Cl-induced prevention of endosomal acidification showed that fusion of MVB that contain virus with lysosomes may lead to the degradation of virus. However, sequestration of infectious virions in MVB indicates that not all MVB that contain HIV-1 fuse with lysosomes; rather, many of them serve as storage for internalized virions. Mechanisms of storage and degradation functions of MVB may be determined by precursors of MVB, which may be formed as a consequence of lipid composition that regulates subsequent fusion of MVB with lysosomes or plasma membranes (Piper and Katzmann, 2007). Indeed, the reduction of HIV-1 sequestration by PI3K and sphingomyelinase inhibitors suggests that precursors of MVB that contain HIV-1 might have diverse lipid compositions. This is consistent with the various internalization pathways of HIV-1 into tonsil, cervical and foreskin epithelial cells. HIV-1 clathrin- and caveolin/lipid raft-associated endocytosis and macropinocytosis may generate distinct early endosome populations that contain virus, which may contribute to the generation of heterogeneous MVB with storage or degradation functions. HIV-1 macropinocytosis and clathrin-associated endocytosis may also generate macropinosomes/vacuoles that contain virus, which may not associate with early endosomes and MVB and thus form independent vesicular compartments that sequester virions.
Several lines of evidence—including reduction of viral sequestration by inhibition of viral attachment and internalization, resistance of sequestered virus to trypsin, pronase and soluble CD4, and independence of viral sequestration from productive infection—indicate that HIV-1 sequestration in mucosal epithelial cells is not associated with surface-accessible membrane invagination, as seen in dendritic cells and macrophages (Cavrois et al., 2007; Yu et al., 2008).
In summary, we have shown that HIV-1 internalization into mucosal epithelia is a complex process that uses multiple cell surface receptors and internalization pathways (Fig. 8). Initial interaction of virus with HSPG, GalCer and TIM-1 and virus entry by endocytosis and macropinocytosis may determine subsequent sequestration of virions in the vesicles, including MVB and vacuoles. Such intraepithelial reservoirs may play a critical role in HIV mucosal transmission. Development of anti-viral compounds to eliminate vesicles containing HIV-1 and/or to inactivate intravesicular virions in mucosal epithelium may help to prevent HIV-1 mucosal transmission.
4. Materials and methods
4.1. Ethics statement
This study was conducted according to the principles expressed in the Declaration of Helsinki and was approved by the Committee on Human Research of the University of California–San Francisco (IRB approval # H8597-30664-03). All human subjects provided written informed consent for the collection of tissue samples. The parents provided informed consent for all minors.
4.2. Viruses and cells
Laboratory-adapted dual (X4-R5)-tropic HIV-1SF33 and the primary isolates R5-tropic HIV-1SF170 and X4-tropic HIV-192UG029 were grown in activated PBMC, which were obtained from HIV-1-negative donors. PBMC were activated with 2.5 μg/ml phytohemagglutinin (Sigma) and 1 μg/ml interleukin-2 (BD Biosciences) for 3 days. All viral stocks were titered by p24 concentration using HIV-1 p24 ELISA (ELISA p24) (PerkinElmer) according to the manufacturer’s instructions.
Primary tonsil epithelial keratinocytes were established from tonsil tissue from 17 HIV-1-negative children < 5 years of age after routine tonsillectomy. Primary cervical keratinocytes were established from ectocervical tissue specimens from 8 HIV-1-negative donors. Tonsil and cervical tissues were placed in a Dispase solution (50 U/ml) (BD Bioscience) overnight at 4 °C. Mucosal epithelial layers were separated from subepithelial connective tissues, and epithelial cells were dissociated with trypsin (0.25%). Foreskin keratinocytes from three independent donors were purchased from Lonza. The keratinocytes were grown in keratinocyte growth medium (KGM Gold) (Lonza) with 1.3 mM calcium, and the purity of the epithelial cells was confirmed by detection of pan keratin using a cocktail of anti-keratin antibodies (Thermo Fisher Scientific). Only those epithelial cell populations that were 100% positive for keratin were used. Keratinocytes from each tissue were propagated at early passages and frozen in liquid nitrogen.
Polarized cells were established in 12-well Transwell two-chamber permeable filter inserts with 3-μm pore size, as described in our previous work (Tugizov et al., 2003, 2013a, 2013b, 2011; Yasen et al., 2017). The polarity of epithelial cells was verified by immunodetection of the tight junction protein ZO-1 and measurement of TER using a Millicell-ERS voltohmmeter, as described (Tugizov et al., 2003, 2013a, b, 2011).
4.3. HIV-1 attachment, internalization and transcytosis assays
The AP surface of polarized cells was pretreated with mouse antibodies against HSPG (U.S. Biological), GalCer (Millipore) or rabbit antibody to TIM-1 (LSBio) (25 μg/ml of each). One set of cells was treated with a pool of isotype control antibodies (25 μg/ml of each). Cells were washed with PBS, pH 7.0. Dual (X4-R5)-tropic HIV-1SF33, R5-tropic HIV-1SF170 or X4-tropic HIV-192UG029 virions at 20 ng/ml of p24 or 10 ng of p24 per insert containing ~ 3 × 105 cells were added to the AP surface of the cells. Cells were incubated at 4 °C for 2 h and then washed 3 times with cold PBS (pH 7.2) and lysed with 1.0% Triton X-100 buffer (150 mM NaCl, 10 mM Tris/HCl, pH 8.0, and a cocktail of protease inhibitors [Roche]). Surface-bound HIV-1 was measured by p24 ELISA and expressed as a percentage of the initial viral inoculum. To remove attached virions from the cell surface by pronase, cells were washed with cold PBS and incubated with pronase (100 μg/ml) (Roche) for 10 min at 4 °C (Marechal et al., 2001; Nobile et al., 2003). Cells were washed 3 times with cold KGM medium containing 10% fetal calf serum and 0.2 mM phenylmethylsulfonyl fluoride (PMSF) to inactivate pronase (Marechal et al., 2001; Nobile et al., 2003). Cells were then lysed with 1% Triton X-100.
For the HIV-1 internalization assay, polarized cells were incubated with 10 ng/insert of HIV-1 p24 on the AP surface for 2 h at 37 °C in CO2. Cells were washed with phosphate-buffered saline (PBS), pH 7.2, and trypsinized with 0.25% trypsin, which removes uninternalized extracellular virions (Herrera et al., 2016; Marechal et al., 2001). Cells were lysed with 1.0% Triton X-100, and intracellular virus was detected by HIV-1 p24 ELISA. Virus entry was expressed as a percentage of the initial inoculum.
HIV-1 transcytosis assays were performed as described previously (Tugizov et al., 2011). Before each experiment and after the transcytosis assay, we confirmed the polarization of cells by measuring TER. For the transcytosis assay, 10 ng/insert of HIV-1 p24 from cell-free virions was added to AP surfaces. Cells were incubated at 37 °C in CO2 for 4 h, and culture medium from the lower chamber was collected for detection of HIV-1 p24 using ELISA. HIV-1 transcytosis was expressed as a percentage of the initial inoculum.
4.4. Assessing HIV-1 sequestration in polarized epithelial cells
Polarized epithelial cells were incubated with 10 ng/insert of HIV-1 p24 on the AP surface, and cells were incubated at 37 °C in CO2 for 4 h. After 4 h uninternalized virions were removed for HIV-1 sequestration analysis using mild 0.05% trypsin for 4–5 min at room temperature (Kinlock et al., 2014; Tugizov et al., 2012). The integrity of cell polarity after trypsin treatment was confirmed by measuring TER of polarized cells throughout the experiment. Cells were cultured for 3 or 6 days, and culture medium was changed every 3 days. For examination of intracellular virions, cells were trypsinized with 0.25% trypsin. Intracellular virus was determined by ELISA p24 and expressed as a percentage of the initial viral inoculum. Each experiment was performed 2–5 times using cells from independent donors with at least triplicate Transwell inserts for each experimental condition.
4.5. Immunofluorescence assay
For immunofluorescence assays, cells or tissue sections were fixed with 4% paraformaldehyde and 2% sucrose in PBS for 5 min, and then permeabilized with 0.01% Triton X-100 in 4% paraformaldehyde for 5 min. For detection of EEA1 and rabankyrin, rabbit antibodies were used (both from Abcam) (1 μg/ml). LAMP1 and LBPA were detected using mouse monoclonal antibodies (Santa Cruz Biotechnology and Millipore, respectively) (1 μg/ml). For detection of HIV-1 p24, we used mouse anti-p24 antibodies (NIH AIDS Research and Reference Reagent Program) (5 μg/ml). Secondary antibodies labeled with DyLight 488, DyLight 594 and Alexa Fluor were purchased from Jackson ImmunoResearch. Cell nuclei were counterstained with TO-PRO-3 iodide or DAPI (blue) (Molecular Probes). The specificity of each antibody was confirmed by negative staining with the corresponding primary isotype control antibody. HIV-1 coentry with dextran-70 was examined by staining of HIV-1 p24 and colocalization with Texas Red-labeled dextran-70 (Thermo Fisher Scientific). Cells were analyzed by using a Leica SP5 laser confocal microscope (Leica Microsystems) or Nikon Eclipse E400 fluorescence microscope (Nikon).
4.6. Western blot assay
Cells were extracted with 1.0% Triton X-100 buffer (150 mM NaCl, 10 mM Tris/HCl, pH 8.0, and a cocktail of protease inhibitors). Proteins were separated on a 4–20% gradient SDS-polyacrylamide gel. For detection of TIM-1, Hrs, rabankyrin, clathrin and caveolin (LSBio, Santa Cruz Biotechnology and Abcam, respectively) were used rabbit and mouse antibodies (5 μg/ml). An equal protein load was confirmed by detection of β-actin (Ambio) (0.5 μg/ml).
4.7. Treatment of cells with pharmacologic agents
First, we determined the nontoxic concentrations of the pharmacologic agents used in this study in polarized epithelial cells using an MTT assay (Biotium). For inhibition of HIV-1 entry, polarized cells were pretreated for 1 h with nontoxic concentrations of chlorpromazine (10 μM), nystatin (12 μg/ml) and 5 (N-ethyl-N-isopropyl) amiloride (100 μM) (all from Sigma). Cells were then washed, and HIV-1SF33 was added to the AP surface of epithelial cells.
For inhibition of HIV-1 replication, cells were treated with 10 μM AZT for 24 h. Cells were exposed to HIV-1 and maintained with AZT for 6 days. For inhibition of MVB formation, cells were treated with LY294002 and/or GW4869 at 5 μM for 24 h and at 1 μM for the next 48 h. For inhibition of acidification of intravesicular compartments, cells were treated with 10, 20 or 30 mM NH4Cl for 2 h, and then cells were washed and HIV-1SF33 was added to the AP surface. Cells were cultured for 2 days with 10, 20 or 30 mM NH4Cl, and intracellular HIV-1 was examined by ELISA p24. The absence of a toxic effect was confirmed for all treatments by using an MTT assay (Biotium).
4.8. Flow cytometry
To examine internalization of rhodamine-labeled dextran-10 or Texas Red-labeled dextran-70 (both from Molecular Probes), cells were treated with dextran for 1 h. Cells were washed and trypsinized, and intercellular dextran was analyzed using flow cytometry on a Becton-Dickenson FACScan flow cytometer. Data were analyzed using FlowJo software (Treestar).
4.9. Domain-selective surface labeling assay
Polarized cells were incubated with 200 μg/ml sulfo-NHS-LC-biotin (Thermo Fisher Scientific) from AP or BL membranes for 30 min as described previously (Sufiawati and Tugizov, 2014; Tugizov et al., 2003; Xiao et al., 2009). Cells were washed with Tris saline (10 mM Tris-HCl, pH 7.4, 120 mM NaCl) and extracted in 1% Triton X-100 lysis buffer containing protease inhibitors. Biotinylated proteins were precipitated with streptavidin–agarose beads in lysis buffer (Thermo Fisher Scientific). Proteins were separated on a 4–20% Tris-glycine SDS-polyacrylamide gel and transferred to nitrocellulose membranes (GE Healthcare). TIM-1 was detected by using mouse monoclonal antibody (Abcam). β-Actin was detected in biotinylated cell extracts, which was used for precipitation of streptavidin–agarose beads. Bands were visualized by using the ECL detection system (GE Healthcare).
4.10. Transfection of cells with small interfering RNAs (siRNAs)
The siRNAs for TIM-1 (sc-61691), Hrs (sc-41232), rabankyrin (sc-93654), caveolin-1 (sc-29241) and clathrin heavy chain (sc-35057), were purchased from Santa Cruz Biotechnology. Unrelated (scrambled) siRNAs (sc-37007) were used as controls. Polarized cells were transfected with siRNAs using lipid-based transfection reagents as described (Kamioka et al., 2010; Tugizov et al., 2011). The efficiency of siRNA transfection was confirmed by using FITC-labeled control siRNA; after 24 h, 70–80% of cells were positive for siRNA-FITC. Silencing of genes of interest by siRNAs at 48 or 72 h after transfection was confirmed by using a Western blot assay with antibodies to TIM-1, Hrs, rabankyrin, clathrin and caveolin. Protein expression was quantified by measuring the intensity of pixels (mean density) in protein bands using Image J software.
4.11. HIV-1 spread assay from epithelial cells to lymphocytes
PBMC were isolated from heparinized blood using a Ficoll-Paque Plus density gradient (Sigma). CD4+ T lymphocytes were then isolated by positive selection using anti-CD4 microbeads (Miltenyi Biotec) (Tugizov et al., 2012). PBMC were activated with 2.5 μg/ml phytohemagglutinin (Sigma) and 1 μg/ml interleukin-2 (BD Biosciences) for 3 days. For cocultivation of activated PBMC with polarized epithelial cells containing HIV, ~ 106 lymphocytes were washed twice and added to AP or BL surfaces of epithelial cells as described in our recent work. The ratio of lymphocytes to epithelial cells was ~ 2:1. After 4 h, medium and lymphocytes were collected by pipeting and centrifuged for 10 min at 1200 rpm. PBMC were grown for 3–12 days and analyzed for HIV-1 infection by ELISA p24.
4.12. Determination of integrated HIV-1 DNA and nonspliced or multispliced viral RNA using quantitative real-time PCR (qPCR)
Polarized epithelial cells containing intracellular HIV-1 were trypsinized to remove extracellular virus. Cell nuclei were isolated using the Nuclei EZ Prep Nuclei Isolation Kit (Sigma). DNA was then extracted from the nuclei using the ArcHIV-1ePure DNA Kit as described by the manufacturer (5 PRIME). Total cellular DNA was quantified by NanoDrop 1000 (Thermo Fisher Scientific); 100 ng of DNA was used for all samples isolated from individual cell lines. In the first reaction, Taq DNA polymerase (Applied Biosystems) was used, and primers (Ferreira et al.) were as follows: Alu-specific sense primer (5′-TCC CAG CTA CTC GGG AGG CTG AGG-3′) and antisense HIV-1-specific primer (M661) (5′-CCT GCG TCG AGA GAT CTC CTC TG-3′, 673–695). Cycling conditions included an initial denaturation step (94 °C for 3 min), followed by 22 cycles of denaturation (94 °C for 30 s), annealing (66 °C for 30 s), and extension (70 °C for 10 min) followed by a final extension (72 °C for 10 min).
To quantify the integrated viral DNA, we used real-time qPCR as described by Suzuki et al. (Ferreira et al., 2015). The first PCR product was subsequently diluted fourfold and subjected to qPCR using the HIV-1 R/U5 region. The Power SYBR PCR Master Mix (total 20 μl) was used, and primers were as follows: R/U5 DNA sense primer (M667) (5′-GGC TAA CTA GGG AAC CCA CTG C-3′, 496–517) and the antisense primer (AA55) (5′-CTG CTA GAG ATT TTC CAC ACT GAC-3′, 612–635). qPCR was performed using the QuantStudio 6 Flex Real-Time PCR System (Thermo Fisher Scientific). The lymphoblastoid cell line 8E5/LAV (NIH AIDS Research and Reference Reagent Program) containing a single integrated copy of proviral DNA per cell served as a positive control (Liszewski et al., 2009).
To detect nonspliced and multispliced HIV-1, we collected total RNA from polarized epithelial cells containing HIV-1. RNA was isolated by using the RNeasy Mini Kit (Qiagen) and quantified by NanoDrop 1000 (Thermo Fisher Scientific). Samples were treated with DNase using Turbo DNA-Free (Ambion). Total RNA (500 ng) was transcribed to cDNA using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). In separate reactions the cDNA was incubated with primers specific for nonspliced (5′-GAC GCT CTC GCA CCC ATC TC-3′ and 5′-CTG AAG CGC GCA CGG CAA-3′) and multispliced (5′-GAC TCA TCA AGT TTC TCT ATC AAA-3′ and 5′-AGT CTC TCA AGC GGT GGT-3′) HIV-1 RNAs.
PCR products were compared to RNA of the housekeeping gene β-actin, which was amplified using the following primers: 5′ATGGCCACGGCTGCTTCCAGC3′ and 5′CATGGTGGTGCCGCCAGACAG3′. The relative expression of RNA was calculated using the delta delta Ct method by relative quantification with QuantStudio 6 Flex software (Thermo Fisher Scientific). Chronically infected 8E5/LAV cells were used as a positive control for all PCR assays.
Acknowledgments
We thank Warner Greene, Marielle Cavrois and Joel Palefsky for discussion. We thank Karen Smith-Mccune for providing cervical tissue samples and for discussion. This project was supported by the by NIH/NIDCR R01DE023315 grant (to ST) and Natural Science Foundation of China (Grant no: 81160468) (to AY).
Appendix A. Supplementary material
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.virol.2017.12.012.
Footnotes
Statistical analysis
Statistical comparisons were made by a two-tailed Student’s t-test. A p value < 0.05 was considered significant.
References
- Adell MA, Teis D. Assembly and disassembly of the ESCRT-III membrane scission complex. FEBS Lett. 2011;585:3191–3196. doi: 10.1016/j.febslet.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloia RC, Jensen FC, Jensen FC, Curtain CC, Mobley PW, Gordon LM. Lipid composition and fluidity of the human immunodeficiency virus. Proc Natl Acad Sci USA. 1988;85:900–904. doi: 10.1073/pnas.85.3.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloia RC, Tian H, Jensen FC. Lipid composition and fluidity of the human immunodeficiency virus envelope and host cell plasma membranes. Proc Natl Acad Sci USA. 1993a;90:5181–5185. doi: 10.1073/pnas.90.11.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloia RC, Tian H, Jensen FC. Lipid composition and fluidity of the human immunodeficiency virus envelope and host cell plasma membranes. Proc Natl Acad Sci USA. 1993b;90:5181–5185. doi: 10.1073/pnas.90.11.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki N, Hamasaki M, Egami Y, Hatae T. Effect of 3-methyladenine on the fusion process of macropinosomes in EGF-stimulated A431 cells. Cell Struct Funct. 2006;31:145–157. doi: 10.1247/csf.06029. [DOI] [PubMed] [Google Scholar]
- Baleux F, Loureiro-Morais L, Hersant Y, Clayette P, Arenzana-Seisdedos F, Bonnaffe D, Lortat-Jacob H. A synthetic CD4-heparan sulfate glycoconjugate inhibits CCR5 and CXCR4 HIV-1 attachment and entry. Nat Chem Biol. 2009;5:743–748. doi: 10.1038/nchembio.207. [DOI] [PubMed] [Google Scholar]
- Batinic D, Robey FA. The V3 region of the envelope glycoprotein of human immunodeficiency virus type 1 binds sulfated polysaccharides and CD4-derived synthetic peptides. J Biol Chem. 1992;267:6664–6671. [PubMed] [Google Scholar]
- Bobardt MD, Chatterji U, Selvarajah S, Van der Schueren B, David G, Kahn B, Gallay PA. Cell-free human immunodeficiency virus type 1 transcytosis through primary genital epithelial cells. J Virol. 2007;81:395–405. doi: 10.1128/JVI.01303-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomsel M. Transcytosis of infectious human immunodeficiency virus across a tight human epithelial cell line barrier. Nat Med. 1997;3:42–47. doi: 10.1038/nm0197-42. [DOI] [PubMed] [Google Scholar]
- Bomsel M, Alfsen A. Entry of viruses through the epithelial barrier: pathogenic trickery. Nat Rev Mol Cell Biol. 2003;4:57–68. doi: 10.1038/nrm1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch ML, Schmidt A, Agy MB, Kimball LE, Morton WR. Infection of Macaca nemestrina neonates with HIV-1 via different routes of inoculation. AIDS. 1997;11:1555–1563. doi: 10.1097/00002030-199713000-00003. [DOI] [PubMed] [Google Scholar]
- Callahan MK, Popernack PM, Tsutsui S, Truong L, Schlegel RA, Henderson AJ. Phosphatidylserine on HIV envelope is a cofactor for infection of monocytic cells. J Immunol. 2003;170:4840–4845. doi: 10.4049/jimmunol.170.9.4840. [DOI] [PubMed] [Google Scholar]
- Campbell SM, Crowe SM, Mak J. Lipid rafts and HIV-1: from viral entry to assembly of progeny virions. J Clin Virol. 2001;22:217–227. doi: 10.1016/s1386-6532(01)00193-7. [DOI] [PubMed] [Google Scholar]
- Carias AM, McCoombe S, McRaven M, Anderson M, Galloway N, Vandergrift N, Fought AJ, Lurain J, Duplantis M, Veazey RS, Hope TJ. Defining the interaction of HIV-1 with the mucosal barriers of the female reproductive tract. J Virol. 2013;87:11388–11400. doi: 10.1128/JVI.01377-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter GC, Bernstone L, Baskaran D, James W. HIV-1 infects macrophages by exploiting an endocytic route dependent on dynamin, Rac1 and Pak1. Virology. 2011;409:234–250. doi: 10.1016/j.virol.2010.10.018. [DOI] [PubMed] [Google Scholar]
- Carter GC, Bernstone L, Sangani D, Bee JW, Harder T, James W. HIV entry in macrophages is dependent on intact lipid rafts. Virology. 2009;386:192–202. doi: 10.1016/j.virol.2008.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavrois M, Neidleman J, Kreisberg JF, Greene WC. In vitro derived dendritic cells trans-infect CD4 T cells primarily with surface-bound HIV-1 virions. PLoS Pathog. 2007;3:e4. doi: 10.1371/journal.ppat.0030004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazal N, Gerlier D. Virus entry, assembly, budding, and membrane rafts. Microbiol Mol Biol Rev. 2003;67:226–237. doi: 10.1128/MMBR.67.2.226-237.2003. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson HC, Belting M. Heparan sulfate proteoglycan as a cell-surface endocytosis receptor. Matrix Biol. 2014;35:51–55. doi: 10.1016/j.matbio.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Commisso C, Flinn RJ, Bar-Sagi D. Determining the macropinocytic index of cells through a quantitative image-based assay. Nat Protoc. 2014;9:182–192. doi: 10.1038/nprot.2014.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell BJ, Lortat-Jacob H. Human immunodeficiency virus and heparan sulfate: from attachment to entry inhibition. Front Immunol. 2013;4:385. doi: 10.3389/fimmu.2013.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen PJ, Korswagen HC. Sorting nexins provide diversity for retromer-dependent trafficking events. Nat Cell Biol. 2012;14:29–37. doi: 10.1038/ncb2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daecke J, Fackler OT, Dittmar MT, Krausslich HG. Involvement of clathrin-mediated endocytosis in human immunodeficiency virus type 1 entry. J Virol. 2005;79:1581–1594. doi: 10.1128/JVI.79.3.1581-1594.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh MH, Anderson MR, McRaven MD, Cianci GC, McCoombe SG, Kelley ZL, Gioia CJ, Fought AJ, Rademaker AW, Veazey RS, Hope TJ. Visualization of HIV-1 interactions with penile and foreskin epithelia: clues for female-to-male HIV transmission. PLoS Pathog. 2015;11:e1004729. doi: 10.1371/journal.ppat.1004729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrowolski R, De Robertis EM. Endocytic control of growth factor signalling: multivesicular bodies as signalling organelles. Nat Rev Mol Cell Biol. 2012;13:53–60. doi: 10.1038/nrm3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorosko SM, Connor RI. Primary human mammary epithelial cells endocytose HIV-1 and facilitate viral infection of CD4 + T lymphocytes. J Virol. 2010;84:10533–10542. doi: 10.1128/JVI.01263-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echbarthi M, Zonca M, Mellwig R, Schwab Y, Kaplan G, DeKruyff RH, Roda-Navarro P, Casasnovas JM. Distinct trafficking of cell surface and endosomal TIM-1 to the immune synapse. Traffic. 2015;16:1193–1207. doi: 10.1111/tra.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewers H, Helenius A. Lipid-mediated endocytosis. Cold Spring Harb Perspect Biol. 2011;3:a004721. doi: 10.1101/cshperspect.a004721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fader CM, Colombo MI. Autophagy and multivesicular bodies: two closely related partners. Cell Death Differ. 2009;16:70–78. doi: 10.1038/cdd.2008.168. [DOI] [PubMed] [Google Scholar]
- Falcone S, Cocucci E, Podini P, Kirchhausen T, Clementi E, Meldolesi J. Macropinocytosis: regulated coordination of endocytic and exocytic membrane traffic events. J Cell Sci. 2006;119:4758–4769. doi: 10.1242/jcs.03238. [DOI] [PubMed] [Google Scholar]
- Fantini J, Hammache D, Delezay O, Yahi N, Andre-Barres C, Rico-Lattes I, Lattes A. Synthetic soluble analogs of galactosylceramide (GalCer) bind to the V3 domain of HIV-1 gp120 and inhibit HIV-1-induced fusion and entry. J Biol Chem. 1997;272:7245–7252. doi: 10.1074/jbc.272.11.7245. [DOI] [PubMed] [Google Scholar]
- Fernandez-Borja M, Wubbolts R, Calafat J, Janssen H, Divecha N, Dusseljee S, Neefjes J. Multivesicular body morphogenesis requires phosphatidyl-inositol 3-kinase activity. Curr Biol: CB. 1999;9:55–58. doi: 10.1016/s0960-9822(99)80048-7. [DOI] [PubMed] [Google Scholar]
- Ferreira VH, Dizzell S, Nazli A, Kafka JK, Mueller K, Nguyen PV, Tremblay MJ, Cochrane A, Kaushic C. Medroxyprogesterone acetate regulates HIV-1 uptake and transcytosis but not replication in primary genital epithelial cells, resulting in enhanced T-cell infection. J Infect Dis. 2015;211:1745–1756. doi: 10.1093/infdis/jiu832. [DOI] [PubMed] [Google Scholar]
- Fredericksen BL, Wei BL, Yao J, Luo T, Garcia JV. Inhibition of endosomal/lysosomal degradation increases the infectivity of human immunodeficiency virus. J Virol. 2002;76:11440–11446. doi: 10.1128/JVI.76.22.11440-11446.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman GJ, Casasnovas JM, Umetsu DT, DeKruyff RH. TIM genes: a family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunol Rev. 2010;235:172–189. doi: 10.1111/j.0105-2896.2010.00903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganor Y, Zhou Z, Tudor D, Schmitt A, Vacher-Lavenu MC, Gibault L, Thiounn N, Tomasini J, Wolf JP, Bomsel M. Within 1 h, HIV-1 uses viral synapses to enter efficiently the inner, but not outer, foreskin mucosa and engages Langerhans-T cell conjugates. Mucosal Immunol. 2010;3(5):506–522. doi: 10.1038/mi.2010.32. . Epub 2010 Jun 23. [DOI] [PubMed] [Google Scholar]
- Gekonge BN, Schiralli G, Schlegel RA, Henderson AJ. Signal transduction induced by apoptotic cells inhibits HIV transcription in monocytes/macrophages. J Leukoc Biol. 2006;80:953–960. doi: 10.1189/jlb.1105638. [DOI] [PubMed] [Google Scholar]
- Girard M, Mahoney J, Wei Q, van der Ryst E, Muchmore E, Barre-Sinoussi F, Fultz PN. Genital infection of female chimpanzees with human immunodeficiency virus type 1. AIDS Res Hum Retrovir. 1998;14:1357–1367. doi: 10.1089/aid.1998.14.1357. [DOI] [PubMed] [Google Scholar]
- Grassel L, Fast LA, Scheffer KD, Boukhallouk F, Spoden GA, Tenzer S, Boller K, Bago R, Rajesh S, Overduin M, Berditchevski F, Florin L. The CD63-Syntenin-1 complex controls post-endocytic trafficking of oncogenic human papillomaviruses. Sci Rep. 2016;6:32337. doi: 10.1038/srep32337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigorov B, Arcanger F, Roingeard P, Darlix JL, Muriaux D. Assembly of infectious HIV-1 in human epithelial and T-lymphoblastic cell lines. J Mol Biol. 2006;359:848–862. doi: 10.1016/j.jmb.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Gu L, Sims B, Krendelchtchikov A, Tabengwa E, Matthews QL. Differential binding of the HIV-1 envelope to phosphatidylserine receptors. Biochim Biophys Acta. 2017;1859:1962–1966. doi: 10.1016/j.bbamem.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamasaki M, Araki N, Hatae T. Association of early endosomal autoantigen 1 with macropinocytosis in EGF-stimulated A431 cells. Anat Rec A Discov Mol Cell Evol Biol. 2004;277:298–306. doi: 10.1002/ar.a.20027. [DOI] [PubMed] [Google Scholar]
- Hanson PI, Cashikar A. Multivesicular body morphogenesis. Annu Rev Cell Dev Biol. 2012;28:337–362. doi: 10.1146/annurev-cellbio-092910-154152. [DOI] [PubMed] [Google Scholar]
- Harrop HA, Rider CC. Heparin and its derivatives bind to HIV-1 recombinant envelope glycoproteins, rather than to recombinant HIV-1 receptor, CD4. Glycobiology. 1998;8:131–137. doi: 10.1093/glycob/8.2.131. [DOI] [PubMed] [Google Scholar]
- Herrera R, Morris M, Rosbe K, Feng Z, Weinberg A, Tugizov S. Human beta-defensins 2 and -3 cointernalize with human immunodeficiency virus via heparan sulfate proteoglycans and reduce infectivity of intracellular virions in tonsil epithelial cells. Virology. 2016;487:172–187. doi: 10.1016/j.virol.2015.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewlett LJ, Prescott AR, Watts C. The coated pit and macropinocytic pathways serve distinct endosome populations. J Cell Biol. 1994;124:689–703. doi: 10.1083/jcb.124.5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hladik F, Sakchalathorn P, Ballweber L, Lentz G, Fialkow M, Eschenbach D, McElrath MJ. Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity. 2007;26:257–270. doi: 10.1016/j.immuni.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocini H, Becquart P, Bouhlal H, Chomont N, Ancuta P, Kazatchkine MD, Belec L. Active and selective transcytosis of cell-free human immunodeficiency virus through a tight polarized monolayer of human endometrial cells. J Virol. 2001;75:5370–5374. doi: 10.1128/JVI.75.11.5370-5374.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocini H, Bomsel M. Infectious human immunodeficiency virus can rapidly penetrate a tight human epithelial barrier by transcytosis in a process impaired by mucosal immunoglobulins. J Infect Dis. 1999;179(Suppl 3):S448–S453. doi: 10.1086/314802. [DOI] [PubMed] [Google Scholar]
- Huotari J, Helenius A. Endosome maturation. EMBO J. 2011;30:3481–3500. doi: 10.1038/emboj.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura T, Asseldonk EJ, Humphreys BD, Gunaratnam L, Duffield JS, Bonventre JV. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J Clin Investig. 2008;118:1657–1668. doi: 10.1172/JCI34487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura T, Bonventre JV, Bailly V, Wei H, Hession CA, Cate RL, Sanicola M. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 1998;273:4135–4142. doi: 10.1074/jbc.273.7.4135. [DOI] [PubMed] [Google Scholar]
- Ivanov AI. Methods in Molecular Biology. Humana Press; Totowa, NJ 07512 USA: 2008a. [Google Scholar]
- Ivanov AI. Pharmacological inhibition of endocytic pathways: is it specific enough to be useful? Methods Mol Biol. 2008b;440:15–33. doi: 10.1007/978-1-59745-178-9_2. [DOI] [PubMed] [Google Scholar]
- Jemielity S, Wang JJ, Chan YK, Ahmed AA, Li W, Monahan S, Bu X, Farzan M, Freeman GJ, Umetsu DT, Dekruyff RH, Choe H. TIM-family proteins promote infection of multiple enveloped viruses through virion-associated phosphatidylserine. PLoS Pathog. 2013;9:e1003232. doi: 10.1371/journal.ppat.1003232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joag SV, Adany I, Li Z, Foresman L, Pinson DM, Wang C, Stephens EB, Raghavan R, Narayan O. Animal model of mucosally transmitted human immunodeficiency virus type 1 disease: intravaginal and oral deposition of simian/human immunodeficiency virus in macaques results in systemic infection, elimination of CD4 + T cells, and AIDS. J Virol. 1997;71:4016–4023. doi: 10.1128/jvi.71.5.4016-4023.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KS, Petrow-Sadowski C, Bertolette DC, Huang Y, Ruscetti FW. Heparan sulfate proteoglycans mediate attachment and entry of human T-cell leukemia virus type 1 virions into CD4 + T cells. J Virol. 2005;79:12692–12702. doi: 10.1128/JVI.79.20.12692-12702.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovic M, Sharma M, Rahajeng J, Caplan S. The early endosome: a busy sorting station for proteins at the crossroads. Histol Histopathol. 2010;25:99–112. doi: 10.14670/hh-25.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamioka N, Akahane T, Kohno Y, Kuroki T, Iijima M, Honma I, Ohba M. Protein kinase C delta and eta differently regulate the expression of loricrin and Jun family proteins in human keratinocytes. Biochem Biophys Res Commun. 2010;394:106–111. doi: 10.1016/j.bbrc.2010.02.125. [DOI] [PubMed] [Google Scholar]
- Kerr MC, Lindsay MR, Luetterforst R, Hamilton N, Simpson F, Parton RG, Gleeson PA, Teasdale RD. Visualisation of macropinosome maturation by the recruitment of sorting nexins. J Cell Sci. 2006;119:3967–3980. doi: 10.1242/jcs.03167. [DOI] [PubMed] [Google Scholar]
- Kinlock BL, Wang Y, Turner TM, Wang C, Liu B. Transcytosis of HIV-1 through vaginal epithelial cells is dependent on trafficking to the endocytic recycling pathway. PloS One. 2014;9:e96760. doi: 10.1371/journal.pone.0096760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi N, Karisola P, Pena-Cruz V, Dorfman DM, Jinushi M, Umetsu SE, Butte MJ, Nagumo H, Chernova I, Zhu B, Sharpe AH, Ito S, Dranoff G, Kaplan GG, Casasnovas JM, Umetsu DT, Dekruyff RH, Freeman GJ. TIM-1 and TIM-4 glycoproteins bind phosphatidylserine and mediate uptake of apoptotic cells. Immunity. 2007;27:927–940. doi: 10.1016/j.immuni.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondratowicz AS, Lennemann NJ, Sinn PL, Davey RA, Hunt CL, Moller-Tank S, Meyerholz DK, Rennert P, Mullins RF, Brindley M, Sandersfeld LM, Quinn K, Weller M, McCray PB, Jr, Chiorini J, Maury W. T-cell immunoglobulin and mucin domain 1 (TIM-1) is a receptor for Zaire Ebolavirus and Lake Victoria Marburgvirus. Proc Natl Acad Sci USA. 2011;108:8426–8431. doi: 10.1073/pnas.1019030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar RB, Maher DM, Herzberg MC, Southern PJ. Expression of HIV receptors, alternate receptors and co-receptors on tonsillar epithelium: implications for HIV binding and primary oral infection. Virol J. 2006;3:25. doi: 10.1186/1743-422X-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letian T, Tianyu Z. Cellular receptor binding and entry of human papillomavirus. Virol J. 2010;7:2. doi: 10.1186/1743-422X-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Wan T, Wan M, Liu B, Cheng R, Zhang R. The effect of the size of fluorescent dextran on its endocytic pathway. Cell Biol Int. 2015;39:531–539. doi: 10.1002/cbin.10424. [DOI] [PubMed] [Google Scholar]
- Li M, Ablan SD, Miao C, Zheng YM, Fuller MS, Rennert PD, Maury W, Johnson MC, Freed EO, Liu SL. TIM-family proteins inhibit HIV-1 release. Proc Natl Acad Sci USA. 2014;111:E3699–E3707. doi: 10.1073/pnas.1404851111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liszewski MK, Yu JJ, O’Doherty U. Detecting HIV-1 integration by repetitive-sampling Alu-gag PCR. Methods. 2009;47:254–260. doi: 10.1016/j.ymeth.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu NQ, Lossinsky AS, Popik W, Li X, Gujuluva C, Kriederman B, Roberts J, Pushkarsky T, Bukrinsky M, Witte M, Weinand M, Fiala M. Human immunodeficiency virus type 1 enters brain microvascular endothelia by macropinocytosis dependent on lipid rafts and the mitogen-activated protein kinase signaling pathway. J Virol. 2002;76:6689–6700. doi: 10.1128/JVI.76.13.6689-6700.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher D, Wu X, Schacker T, Horbul J, Southern P. HIV binding, penetration, and primary infection in human cervicovaginal tissue. Proc Natl Acad Sci USA. 2005;102:11504–11509. doi: 10.1073/pnas.0500848102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marechal V, Prevost MC, Petit C, Perret E, Heard JM, Schwartz O. Human immunodeficiency virus type 1 entry into macrophages mediated by macropinocytosis. J Virol. 2001;75:11166–11177. doi: 10.1128/JVI.75.22.11166-11177.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey LM, Macara IG. Epithelial organization, cell polarity and tumorigenesis. Trends Cell Biol. 2011;21:727–735. doi: 10.1016/j.tcb.2011.06.005. [DOI] [PubMed] [Google Scholar]
- McCullough J, Colf LA, Sundquist WI. Membrane fission reactions of the mammalian ESCRT pathway. Annu Rev Biochem. 2013;82:663–692. doi: 10.1146/annurev-biochem-072909-101058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer J, Helenius A. Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science. 2008;320:531–535. doi: 10.1126/science.1155164. [DOI] [PubMed] [Google Scholar]
- Mercer J, Helenius A. Virus entry by macropinocytosis. Nat Cell Biol. 2009;11:510–520. doi: 10.1038/ncb0509-510. [DOI] [PubMed] [Google Scholar]
- Mercer J, Knebel S, Schmidt FI, Crouse J, Burkard C, Helenius A. Vaccinia virus strains use distinct forms of macropinocytosis for host-cell entry. Proc Natl Acad Sci USA. 2010:9346–9351. doi: 10.1073/pnas.1004618107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer J, Schelhaas M, Helenius A. Virus entry by endocytosis. Annu Rev Biochem. 2010;79:803–833. doi: 10.1146/annurev-biochem-060208-104626. . Review. [DOI] [PubMed] [Google Scholar]
- Mikulak J, Teichberg S, Faust T, Schmidtmayerova H, Singhal PC. HIV-1harboring renal tubular epithelial cell interaction with T cells results in T cell trans-infection. Virology. 2009;385:105–114. doi: 10.1016/j.virol.2008.11.029. [DOI] [PubMed] [Google Scholar]
- Miller CJ, Li Q, Abel K, Kim EY, Ma ZM, Wietgrefe S, La Franco-Scheuch L, Compton L, Duan L, Shore MD, Zupancic M, Busch M, Carlis J, Wolinsky S, Haase AT. Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus. J Virol. 2005;79:9217–9227. doi: 10.1128/JVI.79.14.9217-9227.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milush JM, Kosub D, Marthas M, Schmidt K, Scott F, Wozniakowski A, Brown C, Westmoreland S, Sodora DL. Rapid dissemination of SIV following oral inoculation. Aids. 2004;18:2371–2380. [PubMed] [Google Scholar]
- Miyauchi K, Kim Y, Latinovic O, Morozov V, Melikyan GB. HIV enters cells via endocytosis and dynamin-dependent fusion with endosomes. Cell. 2009;137:433–444. doi: 10.1016/j.cell.2009.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller-Tank S, Kondratowicz AS, Davey RA, Rennert PD, Maury W. Role of the phosphatidylserine receptor TIM-1 in enveloped-virus entry. J Virol. 2013;87:8327–8341. doi: 10.1128/JVI.01025-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muroyama A, Lechler T. Polarity and stratification of the epidermis. Semin Cell Dev Biol. 2012;23:890–896. doi: 10.1016/j.semcdb.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson DP, West M, Odorizzi G. Did2 coordinates Vps4-mediated dissociation of ESCRT-III from endosomes. J Cell Biol. 2006;175:715–720. doi: 10.1083/jcb.200606113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobile C, Moris A, Porrot F, Sol-Foulon N, Schwartz O. Inhibition of human immunodeficiency virus type 1 Env-mediated fusion by DC-SIGN. J Virol. 2003;77:5313–5323. doi: 10.1128/JVI.77.9.5313-5323.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orenstein JM. Replication of HIV-1 in vivo and in vitro. Ultrastruct Pathol. 2007;31:151–167. doi: 10.1080/01913120701344343. [DOI] [PubMed] [Google Scholar]
- Payne CK, Jones SA, Chen C, Zhuang X. Internalization and trafficking of cell surface proteoglycans and proteoglycan-binding ligands. Traffic. 2007;8:389–401. doi: 10.1111/j.1600-0854.2007.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper RC, Dikic I, Lukacs GL. Ubiquitin-dependent sorting in endocytosis. Cold Spring Harb Perspect Biol. 2014;6 doi: 10.1101/cshperspect.a016808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper RC, Katzmann DJ. Biogenesis and function of multivesicular bodies. Annu Rev Cell Dev Biol. 2007;23:519–547. doi: 10.1146/annurev.cellbio.23.090506.123319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper RC, Luzio JP. Ubiquitin-dependent sorting of integral membrane proteins for degradation in lysosomes. Curr Opin Cell Biol. 2007;19:459–465. doi: 10.1016/j.ceb.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt EJ, Gomes MM, Kabat D. Reversible and efficient activation of HIV-1 cell entry by a tyrosine-sulfated peptide dissects endocytic entry and inhibitor mechanisms. J Virol. 2014;88:4304–4318. doi: 10.1128/JVI.03447-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racoosin EL, Swanson JA. Macropinosome maturation and fusion with tubular lysosomes in macrophages. J Cell Biol. 1993;121:1011–1020. doi: 10.1083/jcb.121.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rider CC, Coombe DR, Harrop HA, Hounsell EF, Bauer C, Feeney J, Mulloy B, Mahmood N, Hay A, Parish CR. Anti-HIV-1 activity of chemically modified heparins: correlation between binding to the V3 loop of gp120 and inhibition of cellular HIV-1 infection in vitro. Biochemistry. 1994;33:6974–6980. doi: 10.1021/bi00188a029. [DOI] [PubMed] [Google Scholar]
- Roderiquez G, Oravecz T, Yanagishita M, Bou-Habib DC, Mostowski H, Norcross MA. Mediation of human immunodeficiency virus type 1 binding by interaction of cell surface heparan sulfate proteoglycans with the V3 region of envelope gp120-gp141. J Virol. 1995;69:2233–2239. doi: 10.1128/jvi.69.4.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Macara IG. Organization and execution of the epithelial polarity programme. Nat Rev Mol Cell Biol. 2014;15:225–242. doi: 10.1038/nrm3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachse M, Urbe S, Oorschot V, Strous GJ, Klumperman J. Bilayered clathrin coats on endosomal vacuoles are involved in protein sorting toward lysosomes. Mol Biol Cell. 2002;13:1313–1328. doi: 10.1091/mbc.01-10-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed MF, Kolokoltsov AA, Albrecht T, Davey RA. Cellular entry of ebola virus involves uptake by a macropinocytosis-like mechanism and subsequent trafficking through early and late endosomes. PLoS Pathog. 2010;6:e1001110. doi: 10.1371/journal.ppat.1001110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saidi H, Magri G, Nasreddine N, Requena M, Belec L. R5- and X4-HIV-1 use differentially the endometrial epithelial cells HEC-1A to ensure their own spread: implication for mechanisms of sexual transmission. Virology. 2007;358:55–68. doi: 10.1016/j.virol.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Schelhaas M. Come in and take your coat off - how host cells provide endocytosis for virus entry. Cell Microbiol. 2010;12:1378–1388. doi: 10.1111/j.1462-5822.2010.01510.x. [DOI] [PubMed] [Google Scholar]
- Schelhaas M, Shah B, Holzer M, Blattmann P, Kuhling L, Day PM, Schiller JT, Helenius A. Entry of human papillomavirus type 16 by actin-dependent, clathrin- and lipid raft-independent endocytosis. PLoS Pathog. 2012;8:e1002657. doi: 10.1371/journal.ppat.1002657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt O, Teis D. The ESCRT machinery. Curr Biol. 2012;22:R116–R120. doi: 10.1016/j.cub.2012.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnatwinkel C, Christoforidis S, Lindsay MR, Uttenweiler-Joseph S, Wilm M, Parton RG, Zerial M. The Rab5 effector Rabankyrin-5 regulates and coordinates different endocytic mechanisms. PLoS Biol. 2004;2:E261. doi: 10.1371/journal.pbio.0020261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serpente N, Hemar A, Cefai D, Dautry-Varsat A, Fagard R, Fischer S, Vaquero C. Transcriptional and post-transcriptional mechanisms are involved in the absence of CD4 surface expression in two HIV-1 chronically infected T cell lines. Int Immunol. 1993;5:939–947. doi: 10.1093/intimm/5.8.939. [DOI] [PubMed] [Google Scholar]
- Setty BN, Betal SG. Microvascular endothelial cells express a phosphatidylserine receptor: a functionally active receptor for phosphatidylserine-positive erythrocytes. Blood. 2008;111:905–914. doi: 10.1182/blood-2007-07-099465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiratsuchi A, Kaido M, Takizawa T, Nakanishi Y. Phosphatidylserine-mediated phagocytosis of influenza A virus-infected cells by mouse peritoneal macrophages. J Virol. 2000;74:9240–9244. doi: 10.1128/jvi.74.19.9240-9244.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan RD, Kuhl BD, Mesplede T, Munch J, Donahue DA, Wainberg MA. Productive entry of HIV-1 during cell-to-cell transmission via dynamin-dependent endocytosis. J Virol. 2013;87:8110–8123. doi: 10.1128/JVI.00815-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobo K, Chevallier J, Parton RG, Gruenberg J, van der Goot FG. Diversity of raft-like domains in late endosomes. PLoS One. 2007;2:e391. doi: 10.1371/journal.pone.0000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Johnston D, Sanson B. Epithelial polarity and morphogenesis. Curr Opin Cell Biol. 2011;23:540–546. doi: 10.1016/j.ceb.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Stahl-Hennig C, Steinman RM, Tenner-Racz K, Pope M, Stolte N, Matz-Rensing K, Grobschupff G, Raschdorff B, Hunsmann G, Racz P. Rapid infection of oral mucosal-associated lymphoid tissue with simian immunodeficiency virus. Science. 1999;285:1261–1265. doi: 10.1126/science.285.5431.1261. [DOI] [PubMed] [Google Scholar]
- Stoddard E, Ni H, Cannon G, Zhou C, Kallenbach N, Malamud D, Weissman D. gp340 promotes transcytosis of human immunodeficiency virus type 1 in Genital tract-derived cell lines and primary endocervical tissue. J Virol. 2009;83:8596–8603. doi: 10.1128/JVI.00744-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sufiawati I, Tugizov SM. HIV-associated disruption of tight and adherens junctions of oral epithelial cells facilitates HSV-1 infection and spread. PloS One. 2014;9:e88803. doi: 10.1371/journal.pone.0088803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugizov SM, Berline JW, Palefsky JM. Epstein-Barr virus infection of polar-ized tongue and nasopharyngeal epithelial cells. Nat Med. 2003;9:307–314. doi: 10.1038/nm830. [DOI] [PubMed] [Google Scholar]
- Tugizov SM, Herrera R, Chin-Hong P, Veluppillai P, Greenspan D, Michael Berry J, Pilcher CD, Shiboski CH, Jay N, Rubin M, Chein A, Palefsky JM. HIV-associated disruption of mucosal epithelium facilitates paracellular penetration by human papillomavirus. Virology. 2013a;446:378–388. doi: 10.1016/j.virol.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugizov SM, Herrera R, Palefsky JM. Epstein-Barr virus transcytosis through polarized oral epithelial cells. J Virol. 2013b;87:8179–8194. doi: 10.1128/JVI.00443-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugizov SM, Herrera R, Veluppillai P, Greenspan D, Soros V, Greene WC, Levy JA, Palefsky JM. HIV is inactivated after transepithelial migration via adult oral epithelial cells but not fetal epithelial cells. Virology. 2011;409:211–222. doi: 10.1016/j.virol.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugizov SM, Herrera R, Veluppillai P, Greenspan D, Soros V, Greene WC, Levy JA, Palefsky JM. Differential transmission of HIV traversing fetal oral/intestinal epithelia and adult oral epithelia. J Virol. 2012;86:2556–2570. doi: 10.1128/JVI.06578-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuma PL, Hubbard AL. Transcytosis: crossing cellular barriers. Physiol Rev. 2003;83:871–932. doi: 10.1152/physrev.00001.2003. [DOI] [PubMed] [Google Scholar]
- Uchil P, Mothes W. Viral entry: a detour through multivesicular bodies. Nat Cell Biol. 2005;7:641–642. doi: 10.1038/ncb0705-641. [DOI] [PubMed] [Google Scholar]
- van den Berg LM, Ribeiro CM, Zijlstra-Willems EM, de Witte L, Fluitsma D, Tigchelaar W, Everts V, Geijtenbeek TB. Caveolin-1 mediated uptake via langerin restricts HIV-1 infection in human Langerhans cells. Retrovirology. 2014;11:123. doi: 10.1186/s12977-014-0123-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandegraaff N, Kumar R, Burrell CJ, Li P. Kinetics of human immunodeficiency virus type 1 (HIV) DNA integration in acutely infected cells as determined using a novel assay for detection of integrated HIV DNA. J Virol. 2001;75:11253–11260. doi: 10.1128/JVI.75.22.11253-11260.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidricaire G, Tremblay MJ. A clathrin, caveolae, and dynamin-independent endocytic pathway requiring free membrane cholesterol drives HIV-1 internalization and infection in polarized trophoblastic cells. J Mol Biol. 2007;368:1267–1283. doi: 10.1016/j.jmb.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Vieira N, Deng FM, Liang FX, Liao Y, Chang J, Zhou G, Zheng W, Simon JP, Ding M, Wu XR, Romih R, Kreibich G, Sun TT. SNX31: a novel sorting nexin associated with the uroplakin-degrading multivesicular bodies in terminally differentiated urothelial cells. PloS One. 2014;9:e99644. doi: 10.1371/journal.pone.0099644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vives RR, Imberty A, Sattentau QJ, Lortat-Jacob H. Heparan sulfate targets the HIV-1 envelope glycoprotein gp120 coreceptor binding site. J Biol Chem. 2005;280:21353–21357. doi: 10.1074/jbc.M500911200. [DOI] [PubMed] [Google Scholar]
- Vogel U, Sandvig K, van Deurs B. Expression of caveolin-1 and polarized formation of invaginated caveolae in Caco-2 and MDCK II cells. J Cell Sci. 1998;111(Pt 6):825–832. doi: 10.1242/jcs.111.6.825. [DOI] [PubMed] [Google Scholar]
- Wang X, Huang W, Liu G, Cai W, Millard RW, Wang Y, Chang J, Peng T, Fan GC. Cardiomyocytes mediate anti-angiogenesis in type 2 diabetic rats through the exosomal transfer of miR-320 into endothelial cells. J Mol Cell Cardiol. 2014;74:139–150. doi: 10.1016/j.yjmcc.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilen CB, Tilton JC, Doms RW. HIV: cell binding and entry. Cold Spring Harb Perspect Med. 2012;2 doi: 10.1101/cshperspect.a006866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollert T, Wunder C, Lippincott-Schwartz J, Hurley JH. Membrane scission by the ESCRT-III complex. Nature. 2009;458:172–177. doi: 10.1038/nature07836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Palefsky JM, Herrera R, Berline J, Tugizov SM. EBV BMRF-2 facilitates cell-to-cell spread of virus within polarized oral epithelial cells. Virology. 2009;388:335–343. doi: 10.1016/j.virol.2009.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasen A, Herrera R, Rosbe K, Lien K, Tugizov SM. Release of HIV-1 sequestered in the vesicles of oral and genital mucosal epithelial cells by epithelial-lymphocyte interaction. PLoS Pathog. 2017;13:e1006247. doi: 10.1371/journal.ppat.1006247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HJ, Reuter MA, McDonald D. HIV traffics through a specialized, surface-accessible intracellular compartment during trans-infection of T cells by mature dendritic cells. PLoS Pathog. 2008;4:e1000134. doi: 10.1371/journal.ppat.1000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Barry de Longchamps N, Schmitt A, Zerbib M, Vacher-Lavenu MC, Bomsel M, Ganor Y. HIV-1 efficient entry in inner foreskin is mediated by elevated CCL5/RANTES that recruits T cells and fuels conjugate formation with Langerhans cells. PLoS Pathog. 2011;7:e1002100. doi: 10.1371/journal.ppat.1002100. [DOI] [PMC free article] [PubMed] [Google Scholar]


