Fig. 4.
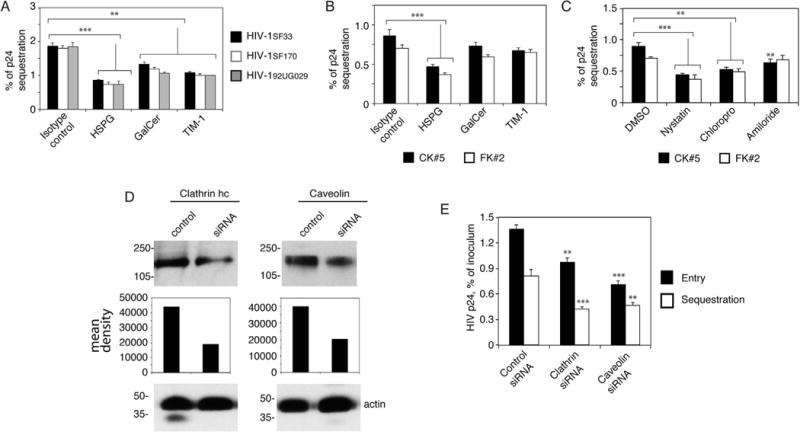
HIV-1 endocytosis and macropinocytosis in tonsil epithelial cells and virus endocytosis in cervical and foreskin epithelial cells play a role in the sequestration of virions in the vesicles. (A) Polarized tonsil epithelial cells were pretreated with antibodies to HSPG, GalCer and TIM-1 for 1 h. Cells treated with isotype antibodies served as a control. Cells were washed and incubated with HIV-1SF33, HIV-1SF170 and HIV-192UG029 viruses for 2 h, washed again, and cultured for 6 days. Cells were collected by trypsinization, and intracellular virions were examined by p24 ELISA. (B) Polarized cervical and foreskin epithelial cells were preincubated with antibodies to HSPG, GalCer and TIM-1. Cells were incubated with HIV-1SF33 and after 6 days were examined for intracellular virus. (C) Cervical and foreskin epithelial cells were treated with chlorpromazine, nystatin, or amiloride for 1 h. Cells were incubated with HIV-1SF33 for 2 h, washed, and after 6 days were examined for intracellular virus. (A-C) Data represent one of two independent experiments and are shown as mean ± SEM of triplicate values. **P < 0.001 and ***P < 0.0001 compared with controls. (D) Tonsil epithelial cells were transfected with clathrin or caveolin siRNA, or control siRNA. After 3 days, clathrin or caveolin expression was examined by Western blot. The mean density of protein bands is shown under the blot. β-Actin was detected in the samples, confirming equal loading. (E) HIV internalization after 2 h and viral sequestration after 3 days were examined in tonsil epithelial cells transfected with clathrin or caveolin siRNA, or control siRNA. Data represent one of two independent experiments and are shown as mean ± SEM of triplicate values. **P < 0.007 and ***P < 0.001 compared with controls.
