Figure 2.
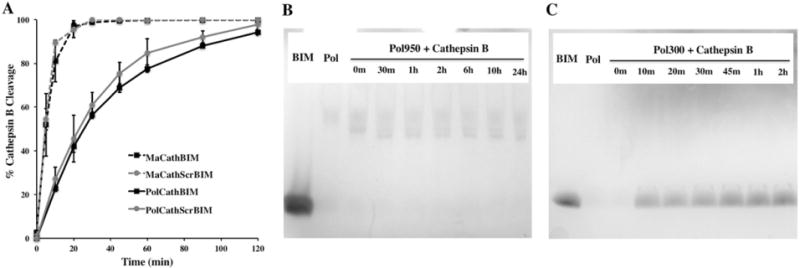
Cathepsin B cleavage of the FKFL peptide linker. (A) Peptide monomers (MaCathBIM and MaCathScrBIM) and diblock copolymers made with PEGMA300 (PolCathBIM and PolCathScrBIM) were incubated with human liver cathepsin B (1.28 ug/mL) in 10 mM phosphate buffer (pH 6.6) at a concentration of 65 μM. At various time points the reactions were stopped by addition of E-64 thioprotease inhibitor (26 ug/mL) and reaction products were analyzed by RP-HPLC and MS. Cathepsin B rapidly and specifically cleaved the FKFL linker to release BIM. (B, C) Protein gel analysis of peptide-PEGMA copolymers (Pol300 and Pol950) following incubation with cathepsin B showed that (B) PEGMA950 hindered cathepsin B access to the FKFL linker, while (C) PEGMA300 permitted cleavage and peptide release from the polymer backbone. For protein gel analyses, enzymatic reactions were run at 3× concentrations and 3.6 nmol of polymer was loaded per well.
