Abstract
Introduction
We analyzed volumetric response of metastatic brain tumors that progressed despite treatment with stereotactic radiosurgery (SRS) after treatment with laser interstitial thermal therapy (LITT).
Methods
We retrospectively reviewed consecutive patients treated from 1/2012 to 10/2015 with LITT for metastatic brain tumors demonstrating progression after SRS. Volumes were quantified using MRI with contrast-enhanced T1-weighted (T1W) and fluid-attenuated inversion recovery (FLAIR).
Results
Fifty lesions from 36 patients were studied. Lesions were assessed prior to LITT, immediately after LITT, 0–90 days after LITT, 90–180 days after LITT, 180–270 days after LITT, and 270–360 days after LITT. The median T1W volume was 5.05cc (range-0.54 to 23.31cc) before LITT treatment (n=50), 7.70cc (range-1.72 to 38.76cc) 0–90 days after LITT (n=47), and 3.68cc (range-1.282 to 48.31cc) 180–270 days after LITT(n=21). The median FLAIR volume was 43.36cc (range-3.09 to 233.01cc) before LITT treatment (n=50), 37.13cc (range- 3.48 to 244.23cc) 0–90 days after LITT (n=43), 31.68cc (range 1.6 to 248.75cc) 180–270 days after LITT (n=18). The 6-month FLAIR volume showed a statistically significant reduction compared to pretreatment (p=0.04). After selecting for cases where patients had two or more post-operative MRIs, we found that 24 lesions (63%) demonstrated an overall downward trend and 14 lesions (37%) demonstrated an upward trend. The median pre-treatment T1W volume for the patients whose lesions demonstrated volumetric reduction after LITT was 3.54cc (range 0.539cc to 10.06cc) and for those who did not demonstrate volumetric reduction after LITT it was 8.81cc (range 0.926cc to 23.313cc).
Conclusion
The pre-treatment tumor volume plays a significant role in determining response to LITT with smaller tumor volumes responding better to LITT than tumors with larger volumes.
Keywords: Laser Interstitial Thermal Therapy, LITT, Metastatic, Post-SRS, Stereotactic radiosurgery, Brain metastasis
Introduction
Laser interstitial thermal therapy (LITT) is being utilized with increasing frequency to treat a variety of brain lesions[1–6]. It is a minimally invasive technique that utilizes the principle of laser-induced hyperthermia to ablate abnormal tissue. One of the most common indications for the use of LITT is lesion progression after stereotactic radiosurgical (SRS) treatment for metastatic brain lesions[7–10]. These progressing lesions may represent treatment failure or radiation necrosis. The incidence of an adverse radiation effect (radiation necrosis) after SRS may be as high as 14% after one year[11]. While radiation necrosis may be self-limiting or responsive to corticosteroids, there is a population of patients who will experience necrosis and edema that is refractory. These patients may be treated with bevacizumab although the benefit will subside after treatment is discontinued [12]. Given that SRS is a common treatment modality for metastatic brain tumors, management of patients with radiation necrosis is a significant issue. LITT is appealing for the treatment of radiation necrosis because its effects on tissue are agnostic to the underlying histology (either tumor or necrosis). LITT induces increased temperature resulting in degradation of tissue at temperatures greater than 43°C [6]. Immediately after the ablation, tissue begins to swell and cerebral edema increases which can make it difficult to ascertain a favorable response to the treatment. In a long-term follow up study evaluating the MRI appearance of brain tumors treated with LITT it was demonstrated that over time, lesion size decreases exponentially[13]. In the present study, we studied the volumetric response over time of post-SRS lesions treated with LITT. Here, we review, to our knowledge, the largest series to date of patients treated with LITT for progression of a metastatic lesion after treatment with SRS.
Methods
Patient Selection
We reviewed a series of consecutive patients undergoing LITT for the treatment of brain metastases that had progressed radiographically (based on routine follow up MR imaging) after treatment with SRS. All patients treated demonstrated progression of their lesions after SRS consistent with either tumor progression, radiation necrosis, or a mixture of both. Demographic information, underlying cancer diagnosis, and performance status were recorded. The treatment dates were from 1/2012 to 10/2015.
Operative Technique
All procedures were performed in an intraoperative MRI suite with a Siemens Espree 1.5T bore (Siemens, Berlin, Germany). LITT procedures were performed by either S.P. or G.R. with either the Visualase (Medtronic, Minneapolis, USA) or Neuroblate (Monteris, Winnipeg, Canada) LITT systems. Details regarding the technique used were reported previously[14]. With the Neuroblate system, the enhancing margins of the tumor were treated to the thermal-damage threshold line corresponding with exposure to 43°C for 10 minutes which is sufficient to induce cell death. Depending up on the geometry of the lesion, either the side-fire or diffusion tip was used. With the Visualase system, the thermal damage was assessed by an expanding volume of thermal damage seen with real time MRI scanning after a high temperature limit is set at 90°C near the tip of the applicator. The low temperature limit is set at 47–50°C at the borders of the target area or near critical structures in order to avoid unintended thermal damage. All lesions were treated to a target temperature of at least 46°C throughout the volume of the lesion to ensure cell death. For larger lesions, a single probe was used and advanced or withdrawn for adequate coverage. In some cases, particularly for irregularly shaped lesions, multiple probes were used.
MRI Imaging/Volumetric analysis
All patients underwent MR imaging of the brain prior to their procedure and follow up imaging at regular intervals after treatment. Imaging sequences included T1 pre- and post-contrast, T2 and FLAIR. The MRI images were exported to the Iplannet workstation (BrainLab, Germany). Using this software, the tumors were segmented for volumetric analysis. This process was performed for T1 post-contrast and the T2 FLAIR sequences. The enhancing disease was outlined using the semi-automated segmentation software available on the IPlannet workstation on the T1 post-contrast images for pre- and post-treatment MRI scans. On the pre-operative scan, the margin was the enhancing lesion. On the post-treatment scan, the margin was the enhancement that represented the ablation volume. The FLAIR signal (hyperintense relative to brain) was similarly segmented using the IPlannet software. Single measurements of each lesion were taken and volumes were verified by the authors (V.B., S.P., and G.R). Only patients with sufficient follow up (at least two volumetric MRI scans after treatment) were included in the volumetric analysis. Six time periods, 1) Pre-LITT MRI scan 2) Post-LITT MRI scan 3) 0–90 days 4) 90–180 days 5) 180–270 days 6) 270–365 days, were used to categorize the tumor volumes for both the T1+ contrast images and the T2 FLAIR images after the LITT procedure.
Statistical analysis was performed with Graphpad Prism (GraphPad, La Jolla, USA). The Mann Whitney test was used to compare median volume measurements. A p value of <0.05 was considered significant. The linear regression analysis was performed using TIBCO S-Plus Version 8.2 for Windows (Palo Alto, CA).
Results
Patient Characteristics
A total of 36 patients (with 50 lesions) were included in the study. There were 16 male and 20 female patients in total. The median age of the patients was 51 years (range 28 to 78). The median Karnofsky performance status score pre-operatively was 80 (Range: 80 to 100). The median Karnofsky performance status score postoperatively was 80 (Range: 70 to 90).
Radiation and Treatment Characteristics
The median SRS dose of radiation was 20 Gy (n= 43, range 12Gy to 24 Gy). The median duration between the date of SRS to the date that the patient was treated with LITT was 330 days (Range: 2 to 790 days). Of the 36 patients, 21 had active extra-cranial disease present at the time of LITT. Twenty-seven patients were being treated with steroids pre-operatively. Prior to treatment with LITT, the median duration of corticosteroid use was 17.5 days (Range: 1 to 182 days). The median duration of hospital stay was 1 day (Range: 1 to 15 days). The median number of days of follow up was 51 days (Range: 7 to 126 days). The median progression free survival for the group was 295 days (n= 3, range: 269 days to 538 days). At the time that the study was conducted 24 patients were alive and 12 patients had died (median overall survival has not yet been reached). Of the deceased patients, 3 died of neurologic causes. 1 patient died from progression of the primary cancer and the remaining 8 patients died of unknown causes.
Histology/Tumor Characteristics
The pathology of the tumors in the study included: adenocarcinoma of the lung (8), adenocarcinoma of the breast (12), adenocarcinoma of the esophagus (1), adenocarcinoma of the rectum (1), neuroendocrine lung cancer(2), clear cell carcinoma of the kidney (1), sarcoma (2), melanoma (15), squamous cell carcinoma of the bladder (1), mixed-ductal lobular carcinoma of the breast (3), mixed epithelial cell carcinoma of the ovary (1), bronchoalveolar carcinoma of the lung (1), papillary carcinoma of the lung (1), and adenocarcinoma of the rectum (1). A total of 6 patients had more than one LITT treatment performed. Of these, only one patient had a recurrent lesion in the same location as the prior ablation, which was then treated again with LITT.
Radiographic Appearance/Imaging
Based on the pre-operative MRI scans, only 1 lesion was not associated with findings of edema/FLAIR attenuation. The remaining 49 lesions all had evidence of edema/FLAIR. 23 lesions were located in the frontal lobe, 8 lesions in the parietal lobe, 7 lesions in the temporal lobe, 5 lesions in the occipital lobe, 4 lesions in the cerebellum, and 1 lesion in the thalamus, basal ganglia, and cingulate gyrus each.
LITT Treatment
The Visualase system was used for 42 lesions and the Neuroblate system was used for 8 lesions.
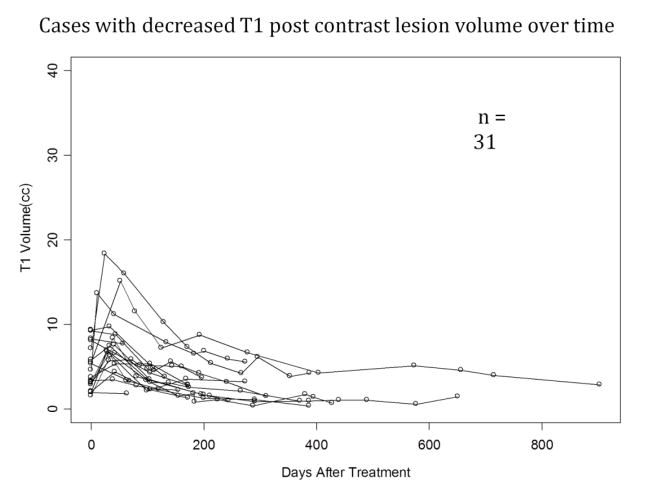
Regression analysis showed that 31 lesions had a downward trend (Figure 1) in T1 post-contrast volumes over time and 19 had an upward trend (Figure 2). We then selected lesions that had two or more post-operative MRIs for volumetric analysis with 24 lesions showing an overall downward trend and 14 lesions showing an upward trend. The 6-month FLAIR volume showed a statistically significant reduction compared to pretreatment levels (p=0.04). The tumor volumes at the pre-specified time points are presented in Tables 1 and 2.
Figure 1.
T1W post contrast lesion volumes plotted against days after LITT procedure. These 31 cases demonstrated a decrease in lesion volume over time.
Figure 2.
T1W (contrast) lesion volumes plotted against days after LITT procedure. These 19 cases demonstrated an increase in lesion volume over time.
Table 1.
Median T1 Weighted MRI with contrast volume over time.
| T1 + C | Pre-LITT N= 50 |
Post-LITT N= 35 |
0–90 days N= 47 |
90–180 days N= 31 |
180–270 days N= 21 |
270–365 days N= 10 |
|---|---|---|---|---|---|---|
| Median (Range) | 5.054cc (0.539 to 23.313) | 7.227cc (0 to 42.497) | 7.697cc (1.721 to 38.762) | 5.153cc (1.539 to 39.93) | 3.677cc (1.282 to 48.307) | 2.6885cc (0.376 to 14.566) |
Table 2.
Median FLAIR volume over time.
| T2 FLAIR | Pre-LITT N= 50 |
Post-LITT N= 22 |
0–90 days N= 43 |
90–180 days N= 27 |
180–270 days N= 18 |
270–365 days N= 10 |
|---|---|---|---|---|---|---|
| Median (Range) | 43.357cc (3.087 to 233.011) | 43.5095cc (8.878 to 218.49) | 37.133cc (3.48 to 244.234) | 26.062cc (0.498 to 311.01) | 31.6835cc (1.598 to 248.749) | 4.679cc (1.361 to 33.98) |
The median pre-treatment T1 post-contrast tumor size for all 50 tumors was 5.05cc (Range- 0.54 to 23.31). The median pre-treatment T1 post-contrast tumor size for the 24 tumors that reduced in volume after treatment was 3.54cc (range 0.54cc to 10.06cc) (Figure 3). The median pre-treatment T1 post-contrast tumor size for the 14 lesions that increased in volume after treatment was 8.81cc (range 0.93cc to 23.31cc) (Figure 4). The difference between these two groups is statistically significant (p-value of 0.012).
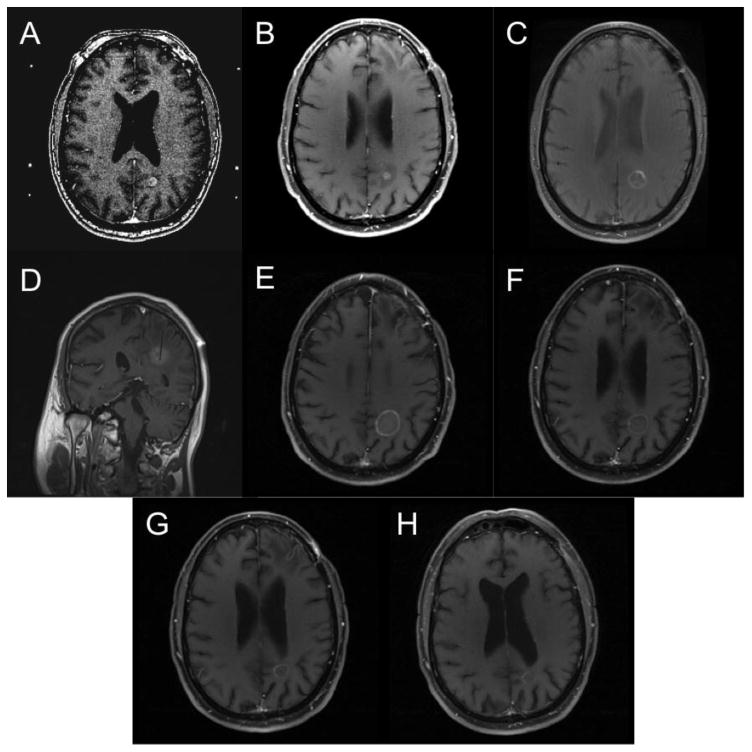
Figure 3.
T1W post contrast MRI scans demonstrating a successful response to LITT over time. A) Pre-SRS demonstrating lesion in the posterior left parieto-occipital region, B) Post-SRS showing initial response, C) Pre-LITT lesion showing enlargement of the lesion. D) Intraoperative placement of laser fiber within the lesion, E) Increase in lesion size immediately Post-LITT. F) One-month post-LITT, G) three months post-LITT, H) Six months post LITT showing near total resolution of the lesion.
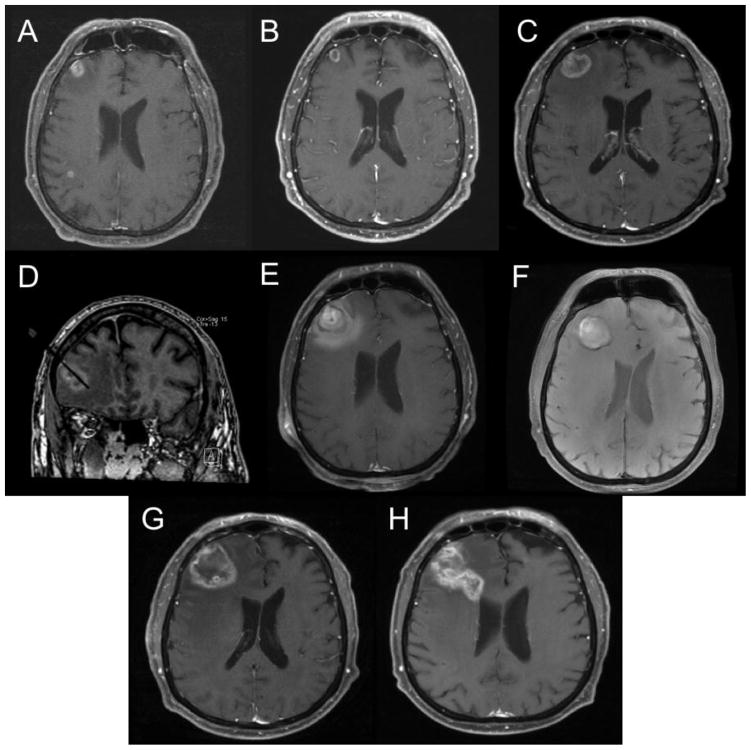
Figure 4.
T1W post contrast MRI scans demonstrating an unsuccessful response to LITT over time. A) Pre-SRS demonstrating lesion in the right frontal region, B) Post-SRS showing initial response. C) Pre-LITT lesion showing enlargement of the lesion. D) Intraoperative placement of laser fiber within the lesion, E) Increase in lesion size immediately Post-LITT. F) One-month post-LITT showing continued enlargement, G) three months post-LITT showing continued enlargement, H) Six months post LITT showing continued enlargement and extension toward the ventricle.
In terms of histology, 9 of the 13 (69.2%) breast cancer lesions demonstrated a reduction in volume after treatment. For the melanoma lesions, 6 of the 11 (54.5%) showed a decrease in volume after treatment. For the lung cancer patients, 4 of the 8 lesions (50%) showed reduced volume after treatment. The sarcoma, bladder, and ovarian cancer lesions all demonstrated a decrease in volume after treatment. The sole esophageal cancer lesion did not demonstrate a reduction in volume after treatment. We also examined median pre-treatment T1 post-contrast tumor size for the three most common pathologies. Lung cancer patients had a median tumor size of 5.69 cc (range- 3.15cc to 9.33cc) compared to melanoma (median- 3.76cc, Range- 0.93cc to 12cc) and breast cancer patients (median- 3.10cc, Range- 1.55cc to 23.31cc).
LITT Failures
A total of 14 lesions demonstrated an increase in lesion volume (based on T1 post contrast images) that was sustained after LITT. One of these failed to respond to a first LITT treatment and was treated a second time with LITT. After failing the second treatment, the patient underwent surgical resection. Three other lesions were treated with resection after demonstrating sustained increase in tumor volume after LITT. Histological examination revealed that two of the resected tumors had active tumor and two were consistent with radiation necrosis. The median pre-operative size of the tumors that required resection was 10.37cc (range 1.55cc to 23.31cc). For the remaining patients, two were treated with further radiation, four either died from their extracranial disease or were transferred to hospice, one patient was lost to follow-up, and one patient, with two lesions, was treated with chemotherapy.
Complications
Sixteen patients (44%) experienced post-operative neurological complications (with some experiencing more than one). Nine patients had motor disturbances, eight patients suffered from an unsteady gait, five patients had visual disturbances, two patients had sensory disturbances, two patients developed aphasia, one patient had difficulty with memory, and one patient developed headaches. Eight patients (50%) were managed expectantly, four patients (25%) were managed with either physical, speech, or occupational therapy, one patient (6.25%) was managed with pain medications and steroids, one patient (6.25%) managed with continued steroids, one patient (6.25%) had an adjustment to their seizure medications, and one patient’s (6.25%) clinical picture was complicated by new metastasis in a different location that was treated with radiosurgery. A total of 9 patients (56.3%) eventually had improvement of their post-operative complications. Of these, 3 patients (33.3%) had improvement in the first month after treatment and 5 patients (55.6%) had improvement at their 3-month follow-up. One patient (11.1%) had improvement at the 5-month follow-up. Four patients (25%) had no change in their post-operative complications at their 1 month and 3 month follow-ups. Two patients (12.5%) developed progressive disease, which made it difficult to assess their neurological status. One patient (6.25%) had progressive worsening of postoperative complications at one and three month follow-up. We identified the anatomic location of the lesion with respect to patients experiencing complications. Five patients had tumors in the left pre central gyrus, and 4 patients had tumors in the right precentral gyrus, and there was 1 patient each who had lesions located in the left thalamus, right occipital lobe, right cerebellum, left parietal, cingulate gyrus, left cerebellum, and left temporal lobe. Out of the 9 lesions located in the precentral gyrus of either hemisphere, 7 patients had difficulty with weakness or gait instability, 1 patient developed an aphasia, and 1 patient developed a headache post-operatively.
Discussion
Laser interstitial thermal therapy (LITT) is a minimally invasive technique that can be used for the treatment of metastatic brain tumors and radiation-induced necrosis[1–6]. The majority of patients with metastatic brain tumors receive some form of radiation as an initial treatment of their disease. Previous studies have shown that this exposure predisposes them to the development of radiation-induced necrosis[11]. A clinical challenge arises when local recurrence of a lesion occurs. This lesion may represent either recurrence of their metastatic tumor or the development of radiation-induced necrosis. The benefit of using LITT is that it is a modality that can treat either necrosis or progressive tumor[8]. Here, we show that LITT is effective for this patient population with a majority of patients experiencing a decrease in the size of the lesion and perilesional edema.
To our knowledge, the present study represents the largest retrospective case series regarding the use of LITT for the treatment of metastatic tumors to the brain that have progressed after SRS. We performed a rigorous volumetric analysis at each imaging time point to correlate tumor volume with outcome [2, 8, 10]. Our results confirm the findings reported in other studies that describe an immediate increase in edema and lesion size after LITT treatment, followed by a gradual decrease and improvement in symptoms.
We found that lesion size increases immediately post-ablation on the T1 post-contrast MRI. This increase in size is temporary, with a reduction in size after 6 months post-treatment. Possible causes for this initial increase in lesion size include inflammatory response and tissue necrosis caused by the ablation[2, 8]. All patients had evidence of extensive FLAIR signal, suggestive of persistent perilesional edema, prior to the LITT procedure. Of these, the majority of treated tumors showed an overall trend showing a decrease in size of FLAIR after treatment with LITT while 14 patients had an overall trend showing increase in size of FLAIR after treatment with LITT. The median FLAIR size decreased over time after the LITT procedure. The overall trend we saw matched the results seen in other studies[8, 10]. The 6-month FLAIR volume showed a statistically significant reduction compared to pretreatment levels. Our results indicate that LITT is particularly effective in reducing the edema associated with these progressive lesions. Although Schwabe et al. reported an exponential reduction in lesion size, the LITT apparatus used was very different and lesion size was restricted due to limitations of the technology at the time. The current available technology permits treatment of much larger and irregular lesions.
The increase in lesion size and edema post-operatively helps explain the relatively high rate of complications that were seen post-operatively. However, these complications were not severe enough to prevent the majority of patients from being discharged within 24 hours. Furthermore, these complications resolved in most cases within 3 months post-operatively, which coincides with the time that the T1 lesion size and edema begins to decrease. The location of the lesion determined the type of complication seen post-operatively. The majority of patients had motor disturbances or gait difficulties post-operatively and the majority of lesions were located in the frontal lobes near primary motor cortex. The eloquent location of these lesions was a reason why LITT was preferred over open craniotomy for treatment.
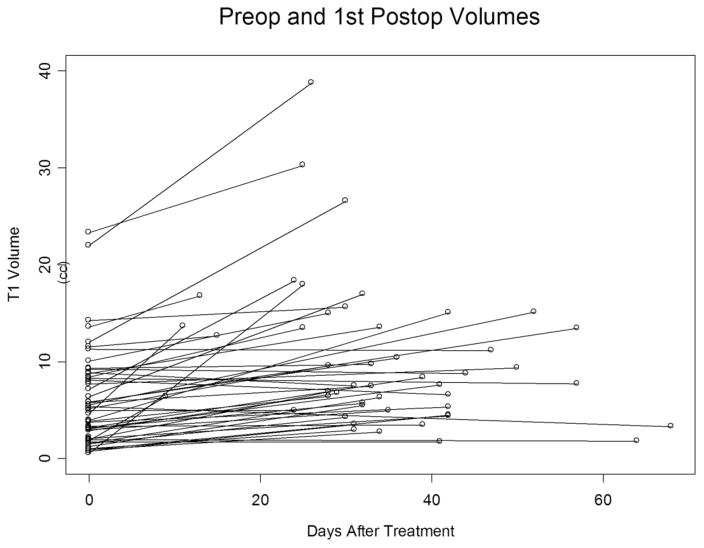
Several parameters were analyzed when looking for an association between treatment success and tumor characteristics including pre-treatment tumor size, tumor histology, and the presence of dural-based lesions. We found that initial tumor size plays a significant role in determining response to LITT (Figure 5). Patients with smaller tumors had better radiographic response than patients with larger tumors. One explanation may be that there may be residual tumor cells left unablated in patients with larger tumors. The finding that patients, who eventually required surgical resection after LITT, had larger tumors than the overall median tumor size further supports this contention. Further studies may determine whether lesion size can be used to segregate patients into a surgical resection treatment arm for larger lesions and LITT for smaller lesions. There were several disparate primary tumor histologies treated in this study. Thus we were unable to determine any correlation between histology and outcome. Given the ablative nature of LITT, the histology of the primary tumor is unlikely to influence response to the treatment.
Figure 5.
Pre-operative and first post-operative T1W post contrast volumes plotted against days after LITT.
A weakness of our study is that at the 6-month post-treatment time point, only 20 of the original 50 lesions were available for analysis. Patients were either deceased or lost to follow up. This is however an inherent difficulty when studying metastatic disease given the poor prognosis.
One possible avenue for future studies would be to examine the difference between the estimated ablation zone and the actual lesion. One possible explanation for the increase seen in 14 lesions lies in the fact that total ablation of the entire lesion may not have been achieved. Each lesion has a unique shape and size while the laser ablation probe creates an ablative field that is cylindrical in shape. Directional probes may help to better contour the thermal ablation to match the lesion geometry.
Conclusion
LITT is an effective option for patients with metastatic brain tumors that have progressed despite prior treatment with stereotactic radiosurgery. Smaller tumor size may predict better response to LITT.
Acknowledgments
Funding: This work was supported by the Cancer Center Support Grant from the National Institutes of Health (P30 CA016672).
Footnotes
Compliance with Ethical Standards:
The authors declare no conflicts of interest.
The study was supported by the Cancer Center Support Grant (P30 CA16672) by the National Institutes of Health.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
The study was approved by the Institutional Review Board (Protocol PA17-0169) which grants a waiver of consent for this retrospective study.
References
- 1.Carpentier A, Chauvet D, Reina V, Beccaria K, Leclerq D, McNichols RJ, Gowda A, Cornu P, Delattre JY. MR-guided laser-induced thermal therapy (LITT) for recurrent glioblastomas. Lasers in surgery and medicine. 2012;44:361–368. doi: 10.1002/lsm.22025. [DOI] [PubMed] [Google Scholar]
- 2.Carpentier A, McNichols RJ, Stafford RJ, Guichard JP, Reizine D, Delaloge S, Vicaut E, Payen D, Gowda A, George B. Laser thermal therapy: real-time MRI-guided and computer-controlled procedures for metastatic brain tumors. Lasers in surgery and medicine. 2011;43:943–950. doi: 10.1002/lsm.21138. [DOI] [PubMed] [Google Scholar]
- 3.Carpentier A, McNichols RJ, Stafford RJ, Itzcovitz J, Guichard JP, Reizine D, Delaloge S, Vicaut E, Payen D, Gowda A, George B. Real-time magnetic resonance-guided laser thermal therapy for focal metastatic brain tumors. Neurosurgery. 2008;63:ONS21–28. doi: 10.1227/01.neu.0000335007.07381.df. discussion ONS28-29. [DOI] [PubMed] [Google Scholar]
- 4.Mohammadi AM, Hawasli AH, Rodriguez A, Schroeder JL, Laxton AW, Elson P, Tatter SB, Barnett GH, Leuthardt EC. The role of laser interstitial thermal therapy in enhancing progression-free survival of difficult-to-access high-grade gliomas: a multicenter study. Cancer medicine. 2014;3:971–979. doi: 10.1002/cam4.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rahmathulla G, Recinos PF, Kamian K, Mohammadi AM, Ahluwalia MS, Barnett GH. MRI-guided laser interstitial thermal therapy in neuro-oncology: a review of its current clinical applications. Oncology. 2014;87:67–82. doi: 10.1159/000362817. [DOI] [PubMed] [Google Scholar]
- 6.Schulze PC, Vitzthum HE, Goldammer A, Schneider JP, Schober R. Laser-induced thermotherapy of neoplastic lesions in the brain--underlying tissue alterations, MRI-monitoring and clinical applicability. Acta neurochirurgica. 2004;146:803–812. doi: 10.1007/s00701-004-0293-5. [DOI] [PubMed] [Google Scholar]
- 7.Rahmathulla G, Recinos PF, Valerio JE, Chao S, Barnett GH. Laser interstitial thermal therapy for focal cerebral radiation necrosis: a case report and literature review. Stereotactic and functional neurosurgery. 2012;90:192–200. doi: 10.1159/000338251. [DOI] [PubMed] [Google Scholar]
- 8.Rao MS, Hargreaves EL, Khan AJ, Haffty BG, Danish SF. Magnetic resonance-guided laser ablation improves local control for postradiosurgery recurrence and/or radiation necrosis. Neurosurgery. 2014;74:658–667. doi: 10.1227/NEU.0000000000000332. discussion 667. [DOI] [PubMed] [Google Scholar]
- 9.Sharma M, Balasubramanian S, Silva D, Barnett GH, Mohammadi AM. Laser interstitial thermal therapy in the management of brain metastasis and radiation necrosis after radiosurgery: An overview. Expert review of neurotherapeutics. 2016;16:223–232. doi: 10.1586/14737175.2016.1135736. [DOI] [PubMed] [Google Scholar]
- 10.Torres-Reveron J, Tomasiewicz HC, Shetty A, Amankulor NM, Chiang VL. Stereotactic laser induced thermotherapy (LITT): a novel treatment for brain lesions regrowing after radiosurgery. Journal of neuro-oncology. 2013;113:495–503. doi: 10.1007/s11060-013-1142-2. [DOI] [PubMed] [Google Scholar]
- 11.Sneed PK, Mendez J, Vemer-van den Hoek JG, Seymour ZA, Ma L, Molinaro AM, Fogh SE, Nakamura JL, McDermott MW. Adverse radiation effect after stereotactic radiosurgery for brain metastases: incidence, time course, and risk factors. Journal of neurosurgery. 2015;123:373–386. doi: 10.3171/2014.10.JNS141610. [DOI] [PubMed] [Google Scholar]
- 12.Levin VA, Bidaut L, Hou P, Kumar AJ, Wefel JS, Bekele BN, Grewal J, Prabhu S, Loghin M, Gilbert MR, Jackson EF. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. International journal of radiation oncology, biology, physics. 2011;79:1487–1495. doi: 10.1016/j.ijrobp.2009.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwabe B, Kahn T, Harth T, Ulrich F, Schwarzmaier HJ. Laser-induced thermal lesions in the human brain: short- and long-term appearance on MRI. Journal of computer assisted tomography. 1997;21:818–825. doi: 10.1097/00004728-199709000-00031. [DOI] [PubMed] [Google Scholar]
- 14.Thomas JG, Rao G, Kew Y, Prabhu SS. Laser interstitial thermal therapy for newly diagnosed and recurrent glioblastoma. Neurosurgical focus. 2016;41:E12. doi: 10.3171/2016.7.FOCUS16234. [DOI] [PubMed] [Google Scholar]