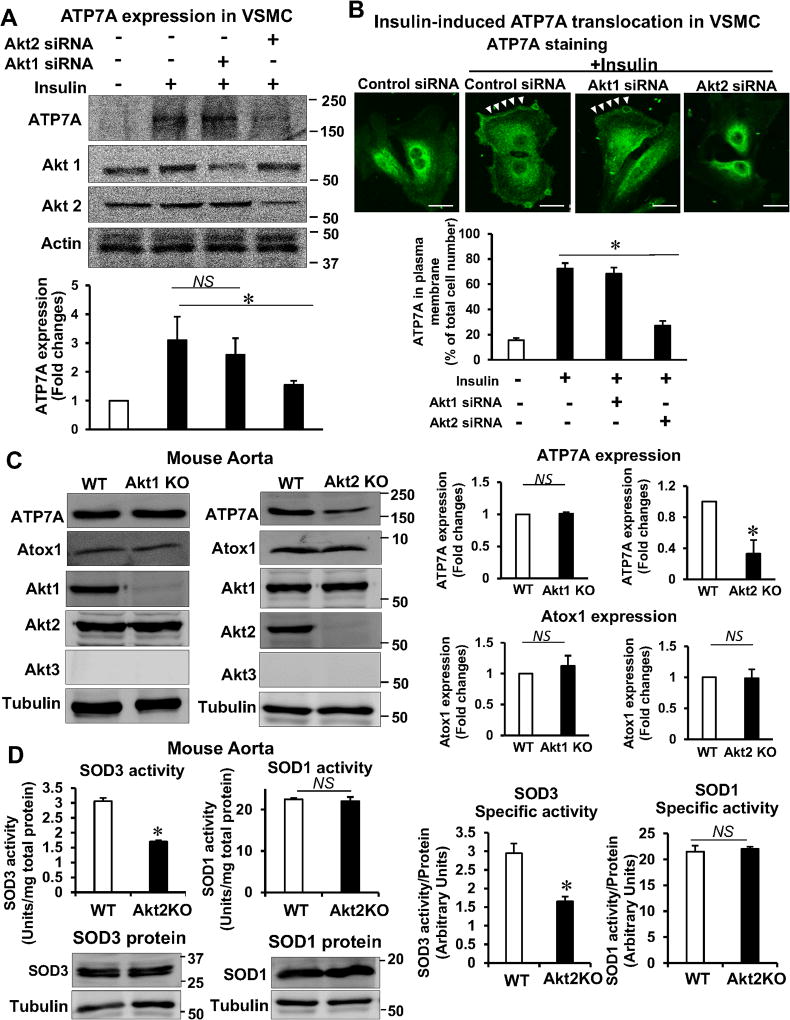
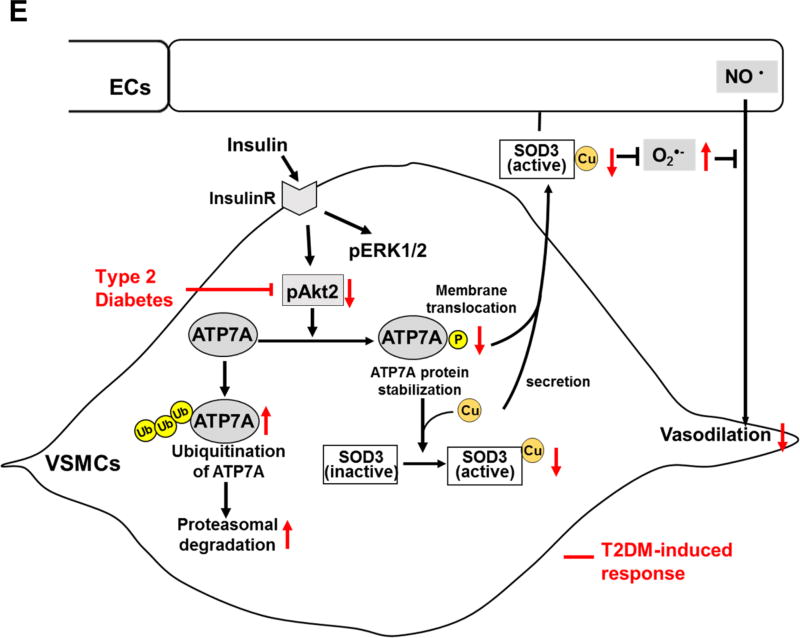
Figure 6. Akt2 is involved in ATP7A protein expression and ATP7A translocation to membrane in VSMCs, which contributes to SOD3 activity.
A and B, RASMs transfected with control or Akt1 or Akt2 siRNA were stimulated with 10 nM insulin for 2 hrs (B) or 16 hrs (A). In A, lysates were used to measure ATP7A protein expression (n=4). In B, cells were stained with anti-ATP7A. Small white stars point to the plasma membrane. Fluorescence images were analyzed as described in Figure 4. Negative control staining lacking the primary antibodies in each staining is shown in Figure XIV in the online-only Data Supplement. C, Protein expression for ATP7A and Atox1 in aortas of female Akt1 or Akt2 KO mice (n=4). D, Specific activity of SOD1 and SOD3 was determined by the ratio of activity to relative amount of protein (n=5). Activities and protein levels of SOD3 and SOD1 in homogenates from female Akt2 KO or WT mice aorta were assayed as described in Figure 2. Results are presented as mean ± SEM. *p<0.05. NS, not significant. E, Proposed model for the protective role of the insulin-Akt2-ATP7A-SOD3 pathway against T2DM-induced endothelial dysfunction. Decreased ATP7A expression in vessels from T2DM with selective impairment of insulin/Akt signaling contributes to decreased SOD3 activity, resulting in increased O2•− production and endothelial dysfunction. Mechanistically, insulin increases Akt2 binding to ATP7A to induce phosphorylation, which may increase ATP7A protein expression via preventing proteasomal degradation as well as ATP7A translocation to the plasma membrane, which contributes to full activation of SOD3 in VSMC and preserves endothelial function.