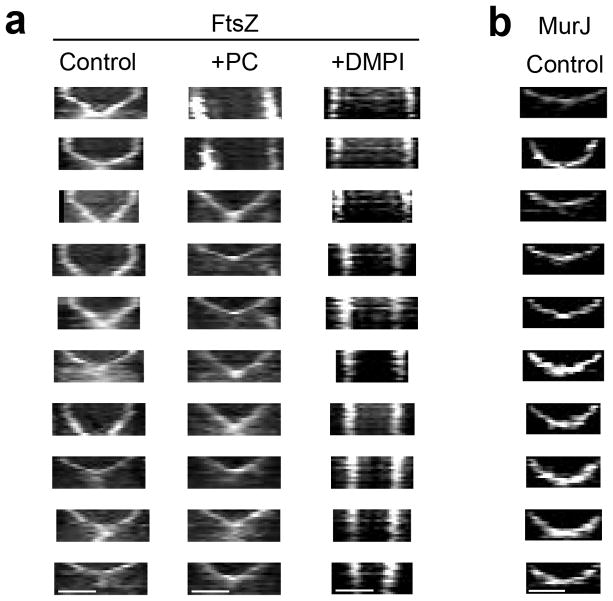
Extended Data Figure 6. Additional kymographs showing constriction of FtsZ55-56sGFP and MurJ-sGFP rings.
a, Kymographs of 10 cells per column showing constriction of FtsZ55-56sGFP rings obtained by imaging ColFtsZ55-56sGFP cells every 5 min for a total of 60 min (laser power 50%), in the absence (Control) or presence of either PC190723 (+PC) or DMPI (+DMPI). Since S. aureus cells are not synchronised, cells at all stages of cytokinesis can be observed. Larger FtsZ55-56sGFP rings had a biphasic behaviour (no/slow constriction followed by fast constriction) while smaller rings, further ahead in the cell cycle, were only observed undergoing the fast constriction step. Addition of PC190723 inhibited constriction of larger rings (see top two kymographs), but not of smaller rings, which were able to complete cytokinesis. Addition of DMPI completely blocked constriction of FtsZ55-56sGFP rings of all sizes. b, Kymographs showing constriction of MurJ-sGFP rings in ColMurJ-sGFP cells imaged every 5 min for a total of 60 min (laser power 100%). Cells where MurJ-sGFP signal appears on the second frame were chosen for analysis to ensure that the entire constriction process was imaged. Fast constriction started immediately upon MurJ-sGFP arrival to the division septum and therefore rings did not show biphasic behaviour. Data are representative of three biological replicates. Scale bars, 0.5 μm.