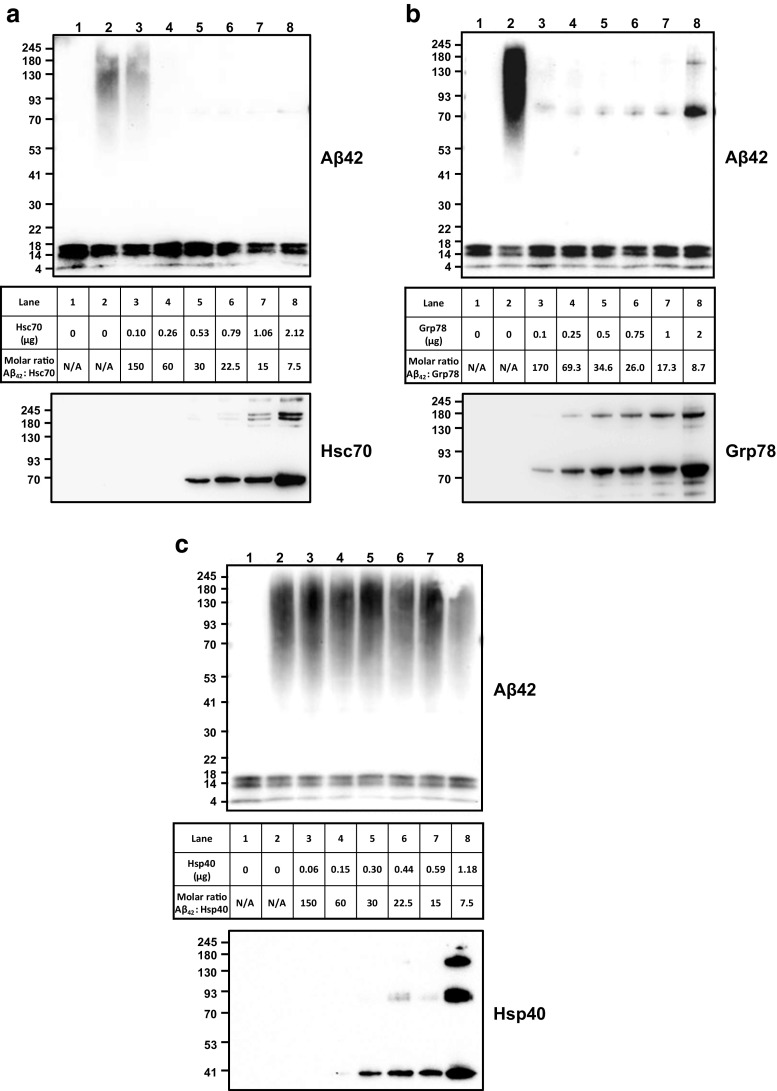
Fig. 8.
Hsc70 and Grp78 interfere with the oligomerization of Aβ peptides. Monomerized Aβ1-42 peptides (1 μg) were kept as a monomer (lane 1), or incubated at 37 °C for 20 h (lane 2), or incubated with various concentrations of a Hsc70 (from 0.1 to 2.12 μg) (lanes 3–8), b Grp78 (from 0.1 to 2 μg) (lanes 3–8), or c Hsp40 (from 0.1 to 1.18 μg) (lanes 3–8), in 4.5 mM Tris pH 7.4 at 37 °C for 24 h. Samples were electrophoresed, transferred onto nitrocellulose membranes, and analyzed by Western blotting using Clone 4G8 antibody followed by an HRP-conjugated secondary antibody. Membranes were stripped and incubated with 1:1000 dilutions of rat anti-Hsc70 (ADI-SPA-815), rabbit anti-Grp78 (StressMarq, SPC-180), or rabbit anti-Hsp40 (Cell Signal # 48685) and the respective secondary HRP-conjugated secondary antibody