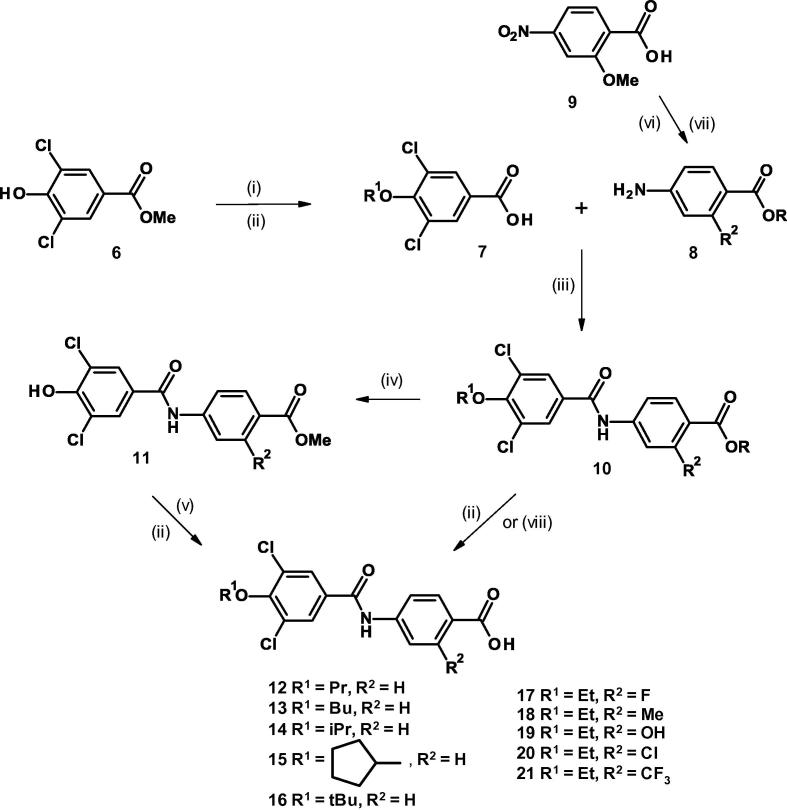
Scheme 1.
4-(3,5-Dichloro-4-alkoxy-benzamido)benzoic acids. (Reagents and conditions: (i) K2CO3, R1Br, DMF, 80 °C, 3 h; (ii) LiOH, THF, H2O, room temp, 12 h; (iii) (COCl)2,CH2Cl2, DMF, 0 °C, 1 h then methyl 4-amino-2-R2-benzoate, NEt3, room temp, 12 h or HATU, DMF, DIPEA, 5 min, then methyl 4-amino-2-R2-benzoate, DMF, room temp, 18 h; (iv) R1 = Bn, R2 = Me; BCl3, CH2Cl2, 0 °C then room temp, 12 h; (v) 1,1-di-tert-butoxy-N,N-dimethylmethanamine, toluene, 80 °C, 3 h, then room temp, 12 h; (vi) 1,1-di-tert-butoxy-N,N-dimethylmethanamine, toluene, 80 °C, 3 h, then further 1,1-di-tert-butoxy-N,N-dimethylmethanamine, 2 mol, added, 80 °C, 16 h. (vii) H2, Pd/C, MeOH, room temp; (viii) R1 = Et, R2 = MeO, R = tBu; BCl3, CH2Cl2, 0 °C then room temp 2 h).