Table 6.
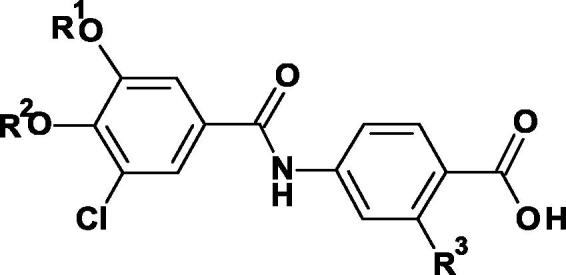
| Potency and Selectivity |
PK |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compd | R1O | R2O | R3 | RARα rel EC50a |
β/α ratio b |
γ/α ratiob |
LogDpH 7.4d | intrinsic Clinte |
rat pof |
|||
| mouse | human | AUC | Cl | F% | ||||||||
| 53 | iPrO | MeO | F | 6.7 | 609 | 17,600 | 1.0 | 11 | 0.3 | 10,871 | 15 | 15 |
| 54 | iPrO | EtO | F | 1.1 | 487 | 6169 | 1.4 | 17.9 | 3.3 | 15,376 | 12 | 12 |
| 43 | iPrO | iPrO | F | 2.7 | 34 | 300 | 2.0 | 18.8 | 6.9 | – | – | – |
| 55 | iPrO | MeO | Me | 4.7c | 241 | 509 | 1.3 | 10.6 | 1.5 | – | – | – |
| 44 | iPrO | iPrO | Me | 0.97 | 45 | 2947 | 2.3 | 26.2 | 15 | 77,670 | 5.4 | 66 |
| 56 | iPrO | EtO | Me | 1.6 | 200 | 11,000 | 1.8 | 37.6 | 5.3 | 70,765 | 7 | 40 |
| 57 | EtO | iPrO | Me | 7 | 286 | 1675 | 1.9 | 25.7 | 8.7 | – | – | – |
| 58 | MeO | EtO | Me | 33 | 210 | >250 | – | 18.4 | – | – | – | – |
| 59 | 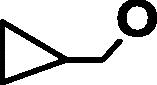 |
EtO | Me | 2.6 | 58 | 38,461 | – | 42.3 | – | – | – | – |
| 45 | tBuO | tBuO | Me | 0.64 | 13,200 | 128 | 2.9 | 36.2 | 28.7 | – | – | – |
