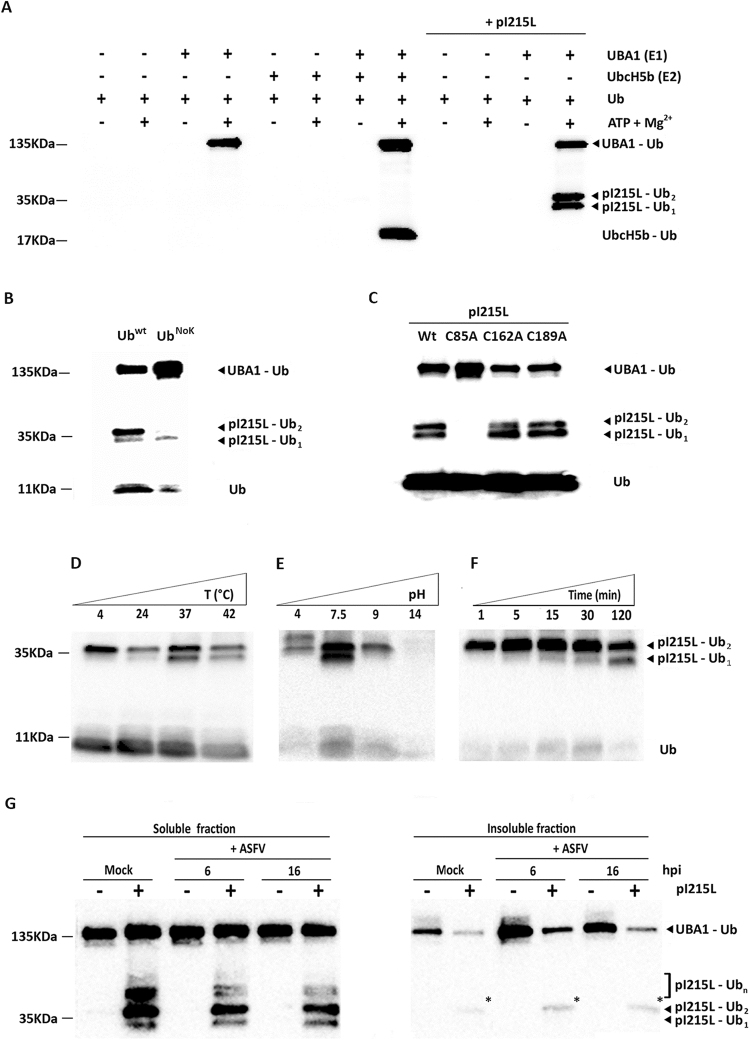
Figure 1.
pI215l acts as an E2-ubiquitin conjugating enzyme. (A) Results from an in vitro ubiquitination assay showed that recombinant pI215L binds one or two ubiquitin molecules, in an ATP- and Mg2+-dependent manner, and in the presence of an E1 ubiquitin-activating enzyme (UBA1). Reaction mixtures were incubated 2 hours at 37 °C, quenched with a non-reducing protein loading buffer, and then subjected to polyacrylamide gel electrophoresis. (B) When the ubiquitination assay was performed using an ubiquitin that is mutated in all lysine residues (UbNoK), the di-ubiquitinated forms of pI215L were not detected. (C) The residue cysteine-85 is essential for the E2-like activity of pI215L. Site-directed mutagenesis revealed that replacement of Cys-85 by an alanine residue led to an absence of ubiquitinated species, whereas the substitution of the Cys-162 or Cys-189 residue does not hamper the formation of ubiquitinated forms of pI215L. (D) pI215L forms thioester bonds with ubiquitin in a wide range of temperatures, although mono-ubiquitinated forms of pI215L were less detectable at 4 °C and 24 °C. (E) pI215L binds ubiquitin in a broad range of pH values, with mono-ubiquitinated forms only found at a pH value of 7.5. (F) Poly-ubiquitinated forms of I215L were detected after a short incubation period of 1 min, whereas the mono-ubiquitinated forms were detected later (5 min), showing increased concentrations in longer incubation times (e.g. 30, 60 minutes). (G) Mono- and poly-ubiquitinated forms of pI215L were mainly found in the Triton X-100-soluble fractions harvested at 6 and 16 hpi. In detergent-insoluble fractions, only the di-ubiquitinated form of pI215L was faintly detected (asterisks). Blots of Fig. 1(D to F) were cropped to improve clarity, full-length blots are presented in supplementary Figure S1. Fig. 1(G) is composed by two individual blots obtained from soluble and insoluble fractions.