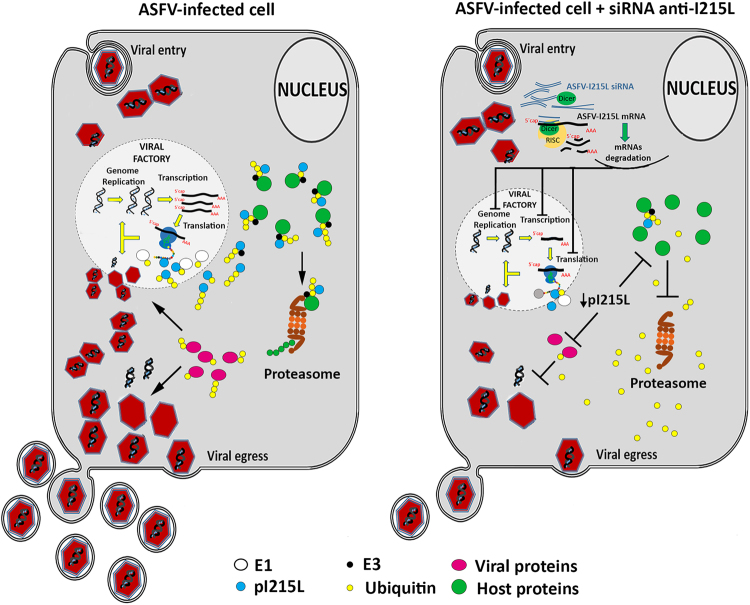
Figure 5.
Proposed working model of the role of pI215L during ASFV infection. Once ASFV enters the host cell, different host mechanisms are subverted in other to generate a productive infection. By encoding an E2 ubiquitin-conjugating enzyme (pI215L), ASFV hijacks the cellular ubiquitin-proteasome system modulating the function and subcellular localization of host proteins and its own proteins. By controlling the ubiquitination status of the cellular proteins, viruses are able to evade host antiviral responses by targeting proteins to proteasomal degradation and to modulate the activity of viral proteins in different mechanisms. Our model suggests that by downregulating I215L expression, a reduction in the abundance of ubiquitin-tagged proteins occurs and consequently causes an inhibition of several crucial viral processes (e.g. genome replication, gene transcription, translation, egress), as well as host pathways (e.g. antiviral immune response, apoptosis).