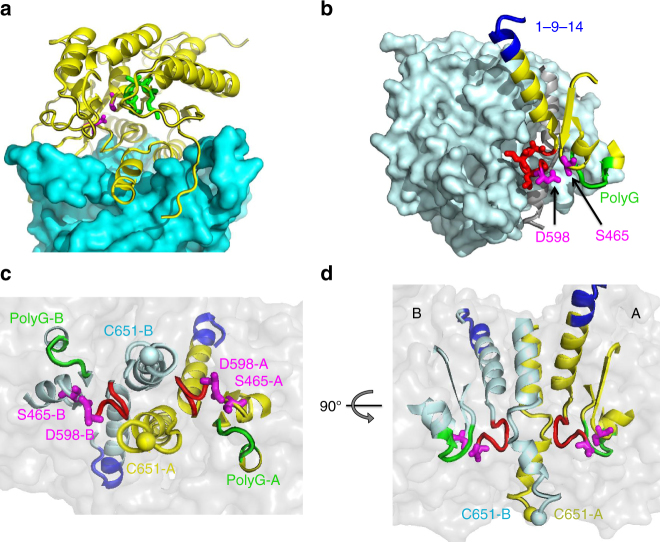
Fig. 3.
Extensive interactions of CAT domains and integrated active sites. a Interaction of the CAT domain of molecule B (CAT-B), shown as cyan surface, with the CAT domain of molecule A (CAT-A), shown as yellow cartoon with highlighted catalytic dyad residues (magenta sticks) and the oxyanion hole (green). b The proximity of the active site to the dimerization interface is illustrated with surface representation of CAT-B (light cyan) and structural elements of the CAT-A active site shown as yellow cartoon, along with the Ser-Asp dyad of CAT-A (magenta stick representation), the oxyanion hole formed by poly-Gly loop (green), and the π-helix (red) which contains the catalytic Asp. The structured fragment of 1-9-14 motif is shown in blue. c The view from the membrane-binding surface of the active sites of a dimer with secondary-structure elements and the individual residues color-coded as in b for molecule A and by light cyan for molecule B. A transparent surface of the dimer is shown in grey. C651 residues of the dimer are represented by yellow and light cyan spheres. These cysteines were previously reported to be acylated in the presence of acyl-CoA and are located on the membrane side of the protein surface. d Side view of the same structural elements in orientation orthogonal to that in c, illustrating the distance of catalytic dyad residues from the membrane-interacting surface and the location of Cys651 at this surface as well as near the dimerization interface