Fig. 5.
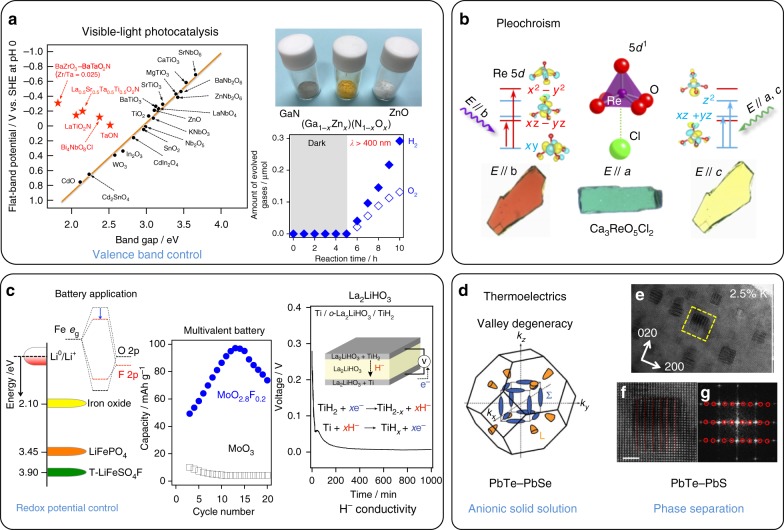
Mixed-anion driven chemical functions. a Visible-light photocatalysis (Fig. 1b). (Left) Flat-band potential as a function of the band gap, showing an empirical relation, EFB(NHE) ≈ 2.94—Eg, for d0 or d10 oxide semiconductors (‘Scaife plot’).51 (Right) Powders of GaN, ZnO and their solid solution (Ga0.58Zn0.42)(N0.58O0.42), and a time course data for overall water splitting under visible light using (Ga0.87Zn0.13)(N0.83O0.16) with RuO2 nanoparticle cocatalyst59. b Pleochroism (Fig. 1a). Ca3ReO5Cl2 crystals showing different optical densities for incident light polarized along the a, b, and c axes68. c Battery applications. (Top left) Energy of the redox couples of iron phosphate frameworks relative to the Fermi level of metallic lithium (Fig. 1a, b)72. (Bottom left) Capacity versus cycle number for MoO2.8F0.2 over the first 18 cycles (Fig. 1b, e)76. (Right) A pure H– conductivity71. Discharge curve for a solid-state battery with the Ti/La2LiHO3/TiH2 structure (Fig. 2a, b). d Thermoelectrics. (Left) Brillouin zone of PbTe1–xSex, where the anion tuning allows creation of low-degeneracy hole pockets (orange) and the high-degeneracy hole pockets (blue)81. (Right) Microstructures for nanoscale precipitates of a phase-segregated (2.5% K-doped) PbTe0.7S0.380. The lower panels show an enlarged image of cubic precipitates with the three-layered structure and its Fourier-transformed image. Some data shown here are reproduced with permission from each journal