Fig. 6.
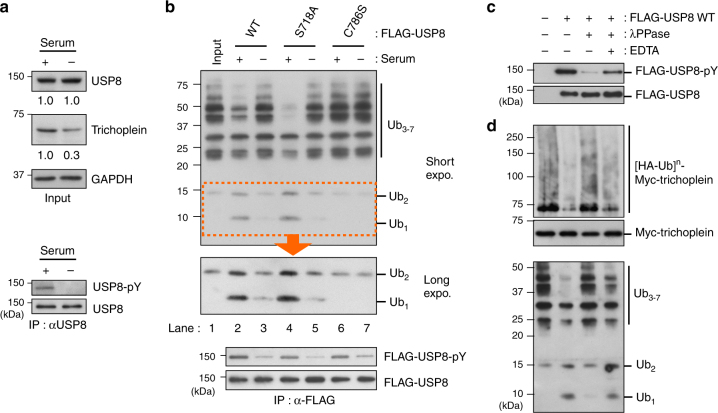
Serum starvation induces dephosphorylation and inactivation of USP8. a USP8 was immunoprecipitated from PRE1 cells cultured in serum-fed medium (serum: + ) or subjected to 24 h serum starvation (serum: -), and the phospho-tyrosine (pY) levels of anti-USP8 immunoprecipitates were analyzed by immunoblotting. The levels of trichoplein, USP8, GAPDH in cell lysates (input) were also analyzed, and normalized intensities of trichoplein/GAPDH and USP8/GAPDH are shown as mean from three independent biological replicates. b FLAG-USP8 variants (WT, S718A, and C786S) were immunopurified from serum-fed (+) or -starved (-) TetOn-RPE1 cells, and then incubated with ubiquitin oligomers (Ub3–7) for 2 h at 37 °C. Anti-ubiquitin, anti-phospho-tyrosine (pY), and anti-FLAG immunoblotting are shown. c FLAG-USP8 WT immunopurified from serum-fed RPE1 cells (lane 2), were treated with λPPase in the absence (lane 3) or presence (lane 4) of EDTA, and analyzed by anti-pY and anti-FLAG immunoblotting. d FLAG-USP8 WT proteins prepared in c were incubated with ubiquitin oligomers (Ub3–7; top) or polyubiquitinated myc-trichoplein ([HA-Ub]n-myc-trichoplein; bottom) for 2 h at 37 °C. Amounts of myc-trichoplein in the reactions are shown in a middle panel