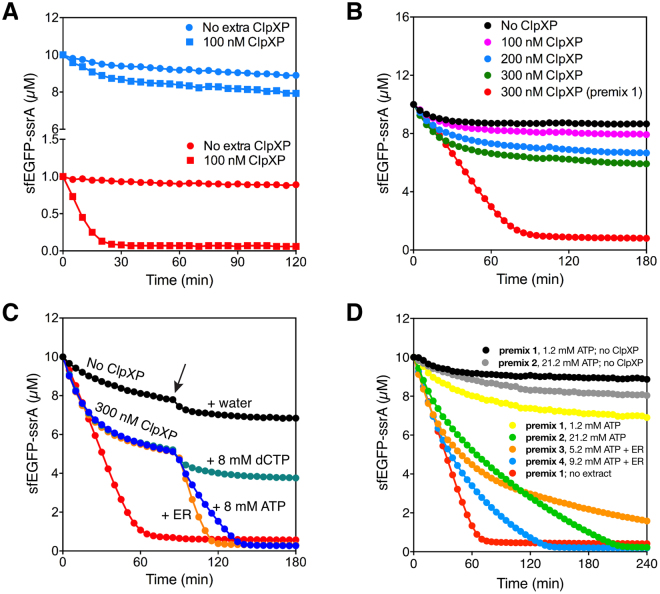
Figure 2.
Optimization of ClpXP degradation in the TX-TL system. (A) ClpXP activity in the TX-TL system. The degradation of 1 μM (red) and 10 μM (blue) sfEGFP-ssrA by 100 nM extra ClpXP added to the complete TX-TL system (square) or by the endogenous ClpXP present in the TX-TL system (circle). In (B), (C) and (D) a positive control reaction was performed with 300 nM ClpXP and 10 µM sfEGFP-ssrA in premix 1 (red). (B) The degradation of sfEGFP-ssrA at different concentrations of ClpXP in the TX-TL system. 10 μM sfEGFP-ssrA were degraded by 0 nM (black), 100 nM (magenta), 200 nM (blue), or 300 nM (green) of ClpXP. (C) Analyzing the effect of increasing the ATP concentration on protein degradation by the wild type ClpXP in the TX-TL system. The degradation of 10 μM sfEGFP-ssrA by 0 nM (black), or 300 nM of ClpXP. After the degradation reactions were incubated for 1.5 hour, either 1 µL of water (magenta), 8 mM dCTP (green), 8 mM ATP (blue) or 8 mM ATP, 16 mM PEP, and 16 U/mL pyruvate kinase (indicated as ER) (orange) solution was added at the time point shown by the arrow. 1 µL of water was also added in negative control reaction (black) at the same time point. (D) Optimization of protein degradation by the wild type ClpXP in the TX-TL system. The degradation of 10 μM sfEGFP-ssrA by 300 nM ClpXP at different concentrations of ATP and in the presence of energy regeneration system. The standard TX-TL system with premix1 (yellow), premix 2 (orange), premix 3 (green), or premix 4 (blue). Control reactions were performed without ClpXP with premix 1 (black) and premix 2 (grey). Positive control reaction (red) was the degradation of 10 μM sfEGFP-ssrA with 300 nM ClpXP in premix 1 without cell extract. The final concentrations of ATP is 1.2 mM in premix 1, 21.2 mM in premix 2, 5.2 mM ATP in premix 3, and 9.2 mM in premix 4. Data are representative of at least three repeated experiments.