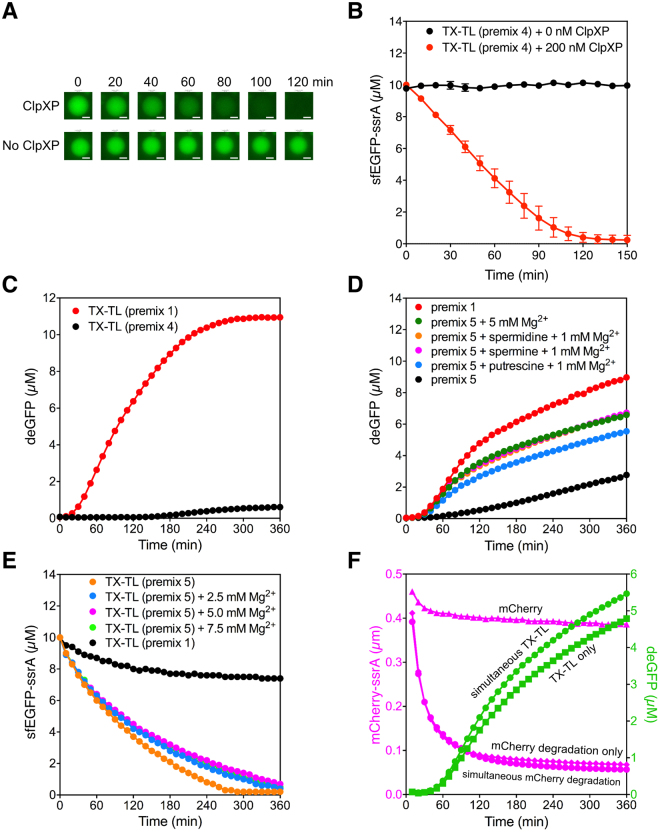
Figure 3.
Degradation of sfEGFP-ssrA in vesicles by ClpXP and optimizing both TX-TL and ClpXP activity. (A) Fluorescence images of sfEGFP-ssrA degradation in a representive vesicle at 0, 20, 40, 60, 80, 100, 120 minutes. The degradation of 10 μM sfEGFP-ssrA in the TX-TL cell extract with 200 nM ClpXP added (top), or no ClpXP added (bottom) are shown. The scale bars represent 10 µm. (B) Time course of ClpXP degradation in vesicles. The degradation kinetics of sfEGFP-ssrA in the presence (red) or absence of ClpXP (black) in vesicles. The degradation kinetics of at least 10 vesicles were measured to calculate the standard deviations. (C) Protein synthesis in the optimized condition for ClpXP activity. Reactions were performed in TX-TL systems containing premix 1 (red) or premix 4 (black). (D) Effects of Mg2+ and polyamines on TX-TL reactions. Reactions were performed under standard condition with premix 5 and additional Mg-glutamate and/or polyamines: 5 mM Mg-glutamate (green); 2 mM spermidine and 1 mM Mg-glutamate (orange); 0.5 mM spermine and 1 mM Mg-glutamate (magenta); 8 mM putrescine and 1 mM Mg-glutamate (blue). Control reactions were performed with premix 1 (red) and premix 5 (black). (E) Effect of additional Mg2+ on ClpXP activity in TX-TL systems. Control reaction (black) was performed in premix 1. Premix 5 was used for all the other reactions. The extra Mg-glutamate added to the reactions were: 0 mM (orange), 2.5 mM (blue), 5 mM (red), and 7.5 mM (green). (F) Simultaneous protein synthesis and degradation in the TX-TL system. All the reactions were performed under standard condition with premix 5 and extra 5 mM Mg-glutamate. Control reactions for protein degradation were performed with 500 nM mCherry-ssrA without ClpXP (pink triangles) or with 300 nM ClpXP (pink diamonds); TX-TL reaction control for deGFP synthesis was performed in the absence of mCherry-ssrA and ClpXP (green squares); combined reactions included degradation of 500 nM mCherry-ssrA by 300 nM ClpXP (pink circles) and deGFP synthesis (green circles). Data are representative of at least three repeated experiments.