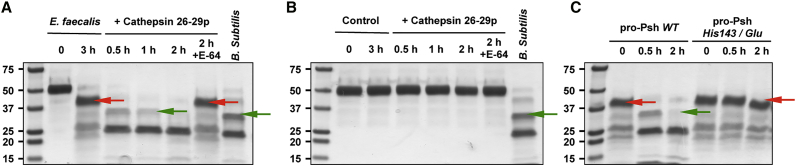
Figure 7.
Sequential Activation of rpro-Psh by Bacterial Proteases and Cathepsin 26-29-p
(A and B) Purified rpro-Psh was incubated in 0.1 M Tris buffer (pH 8) with (A) or without (B) E. faecalis culture supernatant at 29°C. After 3 hr, the partially processed rpro-Psh was incubated with the pre-activated cathepsin 26-29-p for 0.5–2 hr at 29°C in 0.2 M sodium acetate buffer (pH 5.5). The generated Psh hydrolysis products were then visualized by Coomassie blue staining after SDS-PAGE electrophoresis. Incubation under the same conditions with cathepsin 26-29-p pre-inactivated with E-64 was used as control. For comparison, hydrolysis products generated by B. subtilis subtilisine generated as previously described are presented. The N-terminal extremities of the hydrolysis products of interest were determined by N-terminal labeling and mass spectrometry. Red arrows indicate N-terminal extremities localized in the bait region, and the green arrows show the expected N-terminal extremity of the active form of Psh.
(C) Alternatively, the experiment was repeated with rpro-Psh His143/Glu.