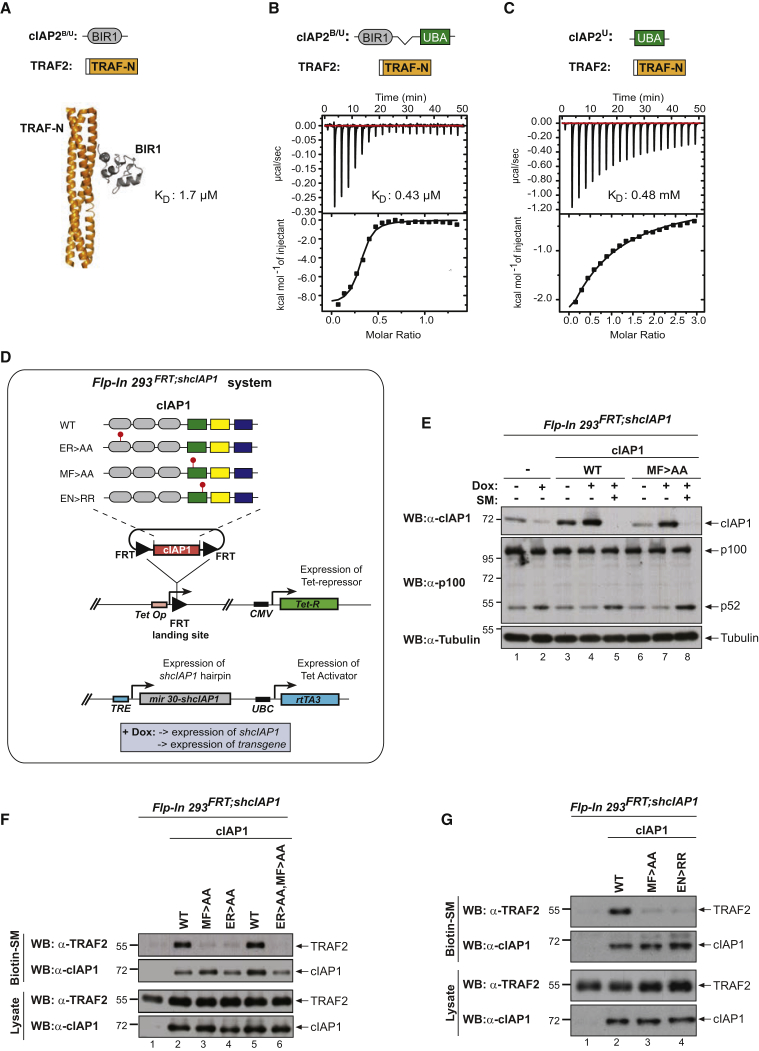
Figure 2.
cIAP2 Requires a Functional UBA Domain to Efficiently Interact with TRAF2
(A–C) Binding of the indicated cIAP2 fragments to TRAF2 was measured by isothermal titration calorimetry. KD, binding constant. Note the data shown in (A) are from Zheng et al. (2010) and are shown for the purposes of comparison only.
(D) Schematic diagram of the Flp-InTMT-RExTM-HEK293shcIAP1 cell system in which endogenous cIAP1 was knocked down via inducible expression of mir30-based shRNA targeting cIAP1’s 3′ UTR. These cells also carry a single FRT site that allows Flp-mediated integration of transgenes into the same transcriptionally regulatable genomic locus. Expression of the transgene and the mir30-based shcIAP1 are induced following treatment with doxycycline (Dox). TRE, tetracycline response element; UBC, ubiquitin promoter; FRT, flippase recognition target; Tet Op, tetracycline operator; Tet-R, tet repressor protein; rtTA3, reverse Tet transactivator (rtTA3).
(E) Western blot analysis of Flp-In cells treated for 72 hr with Dox (100 ng/mL), to allow expression of the indicated transgenes, followed by treatment with the SMAC mimetic (SM) compound A (100 nM) for 6 hr.
(F and G) Biotinylated SM was used to purify IAPs from lysates of Flp-In cells that were treated with Dox for 72 hr. TRAF2-binding was then assessed by immunoblotting. In parallel, expression levels of cIAP1 and TRAF2 were controlled by immunoblotting total cell lysates with the respective antibodies. Representative immunoblots are shown of three independent experiments.