This retrospective medical record review of 54 patients and 74 affected ears examines the association between vestibular aqueduct size and repeated measures of hearing loss.
Abstract
Importance
Elucidating the relationship between vestibular aqueduct size and hearing loss progression may inform the prognosis and counseling of patients who have an enlarged vestibular aqueduct (EVA).
Objectives
To examine the association between vestibular aqueduct size and repeated measures of hearing loss.
Design, Setting, and Participants
For this retrospective medical record review, 52 patients with a diagnosis of hearing loss and radiologic diagnosis of EVA according to the Valvassori criterion were included. All available speech reception threshold and word recognition score data was retrieved; mixed-effects models were constructed where vestibular aqueduct size, age at diagnosis of hearing loss, and time since diagnosis of hearing loss were used to predict repeated measures of hearing ability. This study was performed at an academic tertiary care center.
Exposures
Variable vestibular aqueduct size, age at first audiogram, length of time after first audiogram.
Main Outcomes and Measures
Speech reception threshold (dB) and word recognition score (%) during routine audiogram.
Results
Overall, 52 patients were identified (29 females [56%] and 23 males [44%]; median age at all recorded audiograms, 7.8 years) with a total of 74 ears affected by EVA. Median (range) vestibular aqueduct size was 2.15 (1.5-5.9) mm, and a median (range) of 5 (1-18) tests were available for each patient. Each millimeter increase in vestibular aqueduct size above 1.5 mm was associated with an increase of 17.5 dB in speech reception threshold (95% CI, 7.2 to 27.9 dB) and a decrease of 21% in word recognition score (95% CI, −33.3 to −8.0 dB). For each extra year after a patient’s first audiogram, there was an increase of 1.5 dB in speech recognition threshold (95% CI, 0.22 to 3.0 dB) and a decrease of 1.7% in word recognition score (95% CI, −3.08 to −0.22 dB).
Conclusions and Relevance
Hearing loss in patients with an EVA is likely influenced by vestibular aqueduct midpoint width. When considering hearing loss prognosis, vestibular aqueduct midpoint width may be useful for the clinician who counsels patients affected by EVA.
Key Points
Question
When using repeated measures of hearing test results, is vestibular aqueduct midpoint width correlated to hearing loss?
Findings
In a retrospective study of patients at a tertiary care center, radiologic measurements of vestibular aqueduct midpoint width were significantly associated with hearing loss. Each extra millimeter of width was associated with an increase of 17.5 dB in speech reception threshold and a decrease of 21% in word recognition score.
Meaning
Vestibular aqueduct midpoint width may offer prognostic value for patients who are diagnosed with enlarged vestibular aqueduct.
Introduction
Hearing loss progression and its reported prevalence is heterogenous among the patient population affected by enlarged vestibular aqueduct (EVA). Despite many studies on hearing loss in this patient population, reported onset and degree of hearing loss has been inconsistent. Similarly, patient presentation is heterogenous: EVA may be syndromic, with accompanying Pendred syndrome for example, or nonsyndromic.
Hearing loss progression is as diverse as its presentation, where hearing loss in patients with EVA can be associated with events that affect a preponderance of patients over the course of their lifetime, such as minor head trauma, shifts in barometric pressure, the Valsalva maneuver, or swimming. Consensus is also lacking in the magnitude of the impact of these events, yet delayed onset and early recognition of EVA offer the potential to address such events and possibly reduce hearing loss progression.
The genetics underlying EVA are also varied, arising from a number of mutations in the SLC26A4, KCNJ10, or GJB2 genes. Compared with significant progress in elucidating genetic associations with EVA, clinical approaches to EVA have remained generally unchanged since clinical trials studying surgical occlusion of the vestibular aqueduct or endolymphatic sac were initially conducted.
In the face of such variable manifestation and genetics, a deeper understanding of specific patient characteristics may improve the accuracy of clinical prognosis. Considering the need for a clearer picture of each individual with EVA, the purpose of this study was 2-fold: first, to examine one of the earliest possible predictors of EVA-related hearing loss, size of the vestibular aqueduct, and second, to explore longitudinal audiology test result changes in the patient population with EVA.
Methods
Study Design
The present study is retrospective, longitudinal, and uses repeated-measures data to construct linear mixed-effects models to explore the relationship between vestibular aqueduct size and audiology metrics. A repeated-measures design was chosen for its use evaluating changes in time while simultaneously accounting for the multiple test results belonging to particular individuals. This study was approved by the University Hospitals institutional review board with a waiver of informed consent.
Study Population
All patients who visited a University Hospitals facility between January 2000 and June 2016 with prespecified diagnosis codes and computed tomography (CT) scans of the internal auditory canal (IAC) were identified as candidates for study. Only patients born after implementation of statewide newborn hearing screening tests were included, thus, only patients born in the year 1990 onwards were included.
Diagnosis codes were chosen according to International Classification of Diseases, Ninth Revision (ICD-9) codes used for patients with EVA at this institution in the past, and included codes for sensorineural hearing loss (389.1; range, 389.10-389.18), unspecified congenital anomaly of ear causing impairment of hearing (744.00), congenital anomaly of inner ear causing impairment of hearing (744.05), and unspecified anomaly of the ear (744.3).
A CT scan of the IAC was chosen as the diagnostic test for presence of EVA based on previous literature. Reference to a diagnosis of EVA in radiological notes for imaging procedures was used to identify patients for inclusion. Vestibular aqueducts were measured by a second radiologist for width at the midpoint of the aqueduct, and ears with a midpoint width greater than 1.5 mm on second radiological examination were included in analysis.
Data Collection
Date of birth and audiogram test dates were recorded for the purposes of determining age at first audiogram and time since first audiogram. Unaided speech reception threshold (SRT) and word recognition scores (WRS) at audiologist-determined levels were gathered from electronic medical records; SRT and WRS were chosen because these measures reflect patient hearing ability as it relates to linguistic communication, as opposed to other measures of pure tones.
Only 1 ear per patient was used to prevent bias toward results from those patients with bilateral EVA. For patients with bilateral EVA, an ear was chosen at random by statistical software (R statistical programming language version 3.3.0 [R Foundation]).
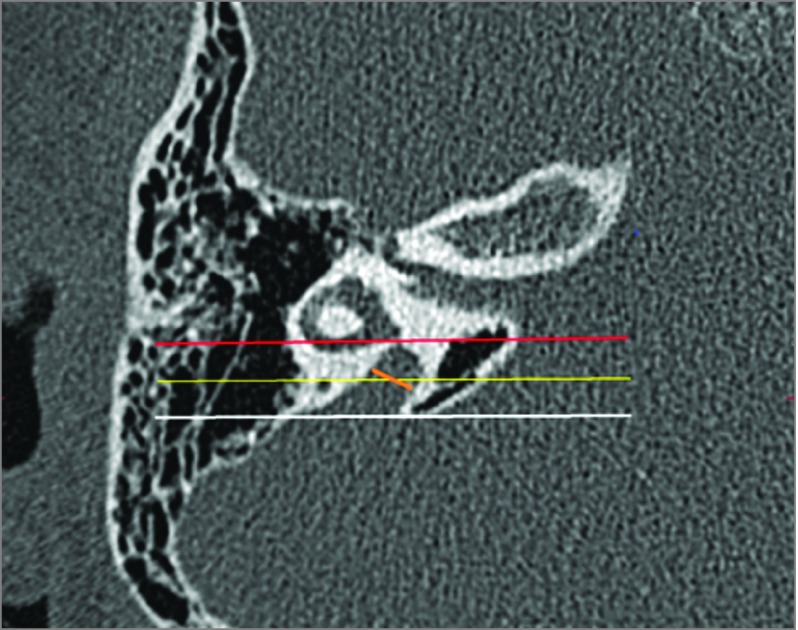
Vestibular aqueduct midpoint was measured using the same procedure as Vijaysekaran et al, where the midpoint of the vestibular aqueduct was defined as (1) halfway between the origin and aperture measured along the length of the vestibular aqueduct or (2) the part of the vestibular aqueduct located half the distance in the petrous bone from its origin in the labyrinth to its aperture in the epidural space. First, an axial slice was chosen where midpoint width appears to be maximum. Next, a line is drawn in coronal plane through posterior wall of the vestibule or crus commune and was used as point of vestibular aqueduct origin, called the vestibular plane. Similarly, a coronal plane through opercular edge defines opening of vestibular aqueduct into the epidural space, called the opercular plane. Following this, a third parallel line is drawn that is parallel to and halfway between the first and second lines, called the midpoint plane. Finally, midpoint measurement is made across the vestibular aqueduct so that it lies equally above and below the midpoint plane and forms the same angle with both walls of the vestibular aqueduct. The above process was repeated at least twice to find the maximum midpoint diameter. This procedure is illustrated in Figure 1.
Figure 1. Axial Computed Tomographic Image of Temporal Bone to Demonstrate Radiological Method.
This axial computed tomographic image of the right temporal bone shows the coronal planes drawn to define the vestibular aqueduct midpoint plane. The vestibular plane (red line) is at the level of the posterior wall of the vestibule. The opercular plane (white line) is at the level of the opercular edge. The midpoint plane (yellow line) is equidistant from the vestibular and opercular planes. The actual measurement line (orange line) is equally above the midpoint plane and forms the same angle with both walls of the vestibular aqueduct.
Statistical Analysis
Longitudinal repeated measures of hearing tests were obtained for each patient so that a linear mixed-effects model could be used to evaluate the relationship between vestibular aqueduct size and 2 outcomes, SRT and WRS. The mixed-effects model was chosen to take advantage of all hearing test measurements for any given patient while at the same time accounting for the grouping of measurements among patients.
Vestibular aqueduct size was the independent variable of interest. Covariates included age at first audiogram measurement to account for recognition of more severe hearing loss at younger ages and time since first audiogram to calculate change in hearing over time.
Two random effects were included: (1) intercepts for each patient and (2) by-subject random slopes over time for the outcome SRT or WRS (Figure 2 and Figure 3).
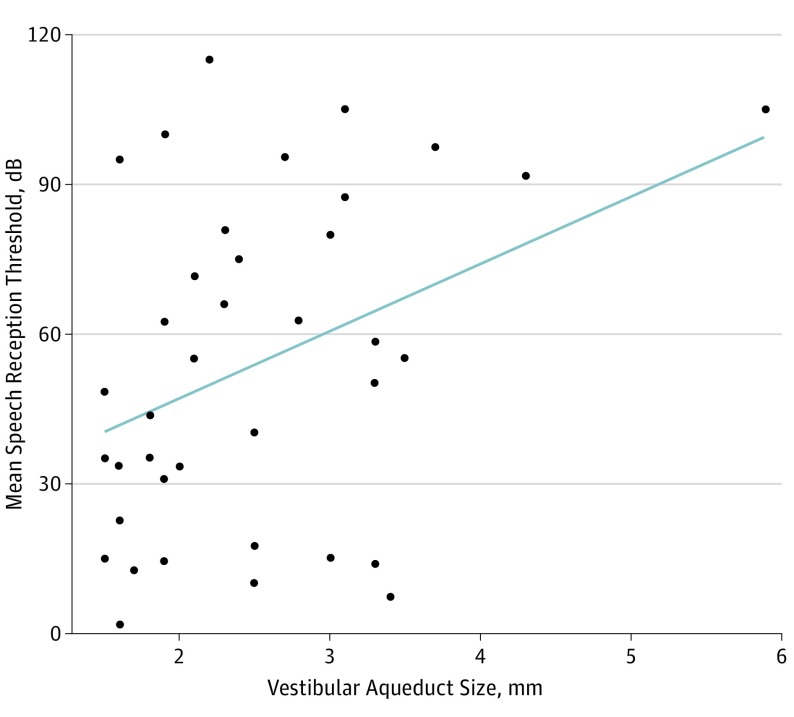
Figure 2. Mean Speech Reception Threshold for Each Patient Compared With Vestibular Aqueduct Size.
The mean speech reception threshold for each patient in relation to their vesitibular aqueduct is shown; a linear regression line is superimposed. The trend is that increased vestibular aqueduct size is associated with an increased speech reception threshold.
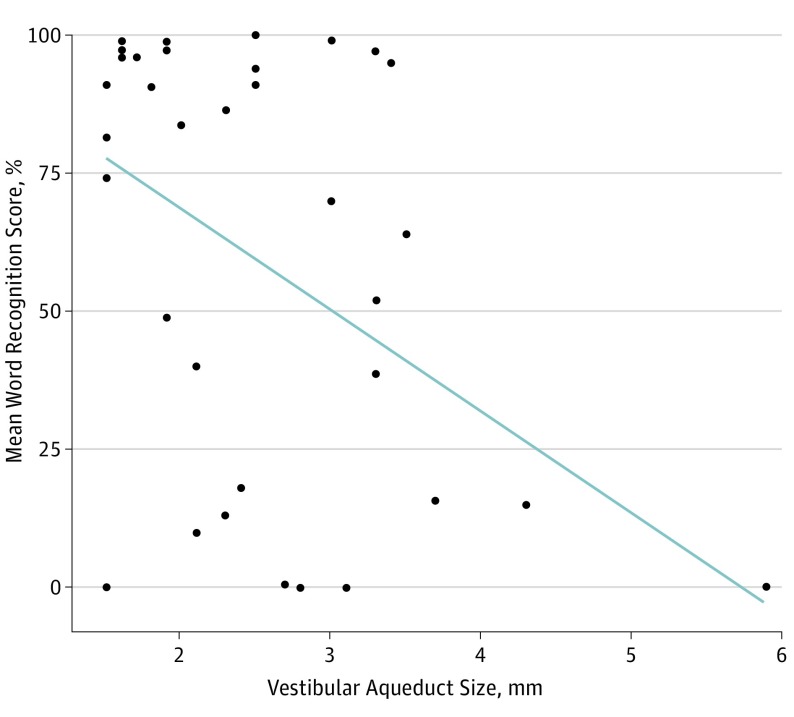
Figure 3. Mean Word Recognition Score for Each Patient Compared With Vestibular Aqueduct Size.
The mean word recognition score compared with vestibular aqueduct size for each patient is shown. The trend is that increased vestibular aqueduct size is associated with decreased mean word recognition score.
Analysis of variance (ANOVA) testing was used to determine the goodness-of-fit of a model with conditional likelihoods compared to a null model with only random effects. Confidence intervals were calculated by computing likelihood profiles and likelihood ratio tests for 10 separate bootstrap iterations. Mixed-effects models were fit by repeated penalized least squares regression using the R statistical programming language version 3.3.0 (R Foundation) software packages lmerTest and lme4.
Linear models were created for comparison with mixed-effects model outcomes, using the mean value of all audiogram results for each patient as predicted by vestibular aqueduct size and age at first audiogram. Bootstrap CIs were produced for linear model coefficients of each audiogram measure. Linear models were produced using the base and car software packages available in the R statistical programming language version 3.3.0 (R Foundation).
Results
Of 365 patients with diagnoses of inner ear malformation or sensorineural hearing loss whose CT scan reports were reviewed, 61 had reports referring to enlarged or slightly enlarged vestibular aqueducts. After a second radiologist measured vestibular aqueduct midpoint width on CT scans, a total of 52 patients (14%) were identified who had EVA according to the Valvassori criterion over the 15 years of selected medical records.
Of the entire cohort of 52 patients (74 ears with EVA; 30 ears without EVA), there were 29 females (56%) and 23 males (44%), with a median age at all recorded audiograms of 7.8 years. Median (range) age at first audiogram was 5.9 (0.0-17.1) years old, and median (range) age at last audiogram was 9.7 (0.7-20.6) years. Median (range) time between audiology visits was 22.4 (0.3-224.0) months.
Median (range) vestibular aqueduct midpoint width among the 52 ears with EVA was 2.2 (1.5-5.9) mm. Mean SRT at first audiogram measurement was 45 dB, while the by-patient mean SRT of all measurements was 53 dB. Mean first available WRS was 64%, and the by-patient mean WRS of all measurements was 60%.
Speech reception threshold measurements were available for 39 of 52 patients, with 195 separate audiogram results available for a median (range) of 5 (1-18) tests per patient. A linear mixed-effects model indicated that the each extra millimeter of vestibular aqueduct midpoint width was associated with a speech reception threshold increase of 17.5 dB (95% CI, 7.2 to 27.9 dB). For each year increase in age at first audiogram, SRT was associated with a decrease of 3.1 dB (95% CI, −5.6 to −0.52 dB); each year after the first audiogram was associated with an increase in SRT of 1.5 dB (95% CI, 0.22 to 3.0 dB).
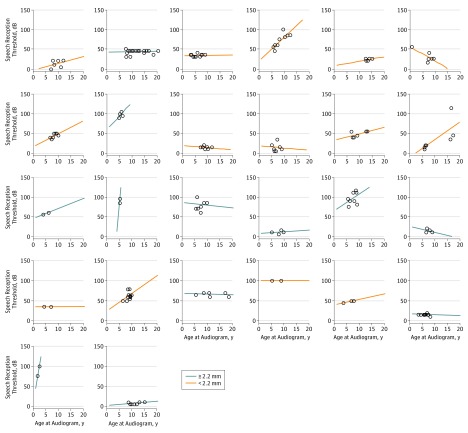
Figure 4 depicts SRT for those patients with 2 or more hearing test results, as individual plots across the range of years among patients.
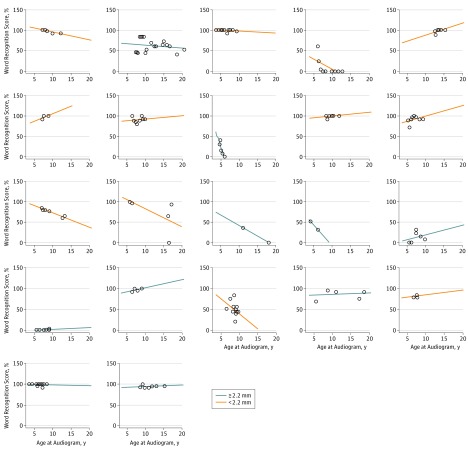
Figure 4. Speech Reception Threshold Test Results Compared With Age of Testing for 26 Patients.
Twenty-six patients with 2 or more hearing test results for speech reception threshold and only the ears randomly selected for numerical analysis are included. Each graph represents an individual patient and shows a regression line of the speech reception threshold compared with the age of the patient at testing; dots represent each test result. Orange lines represent patients with a vestibular midpoint width that is less than the median width (2.2 mm) among the patient population for this study.
According to the linear model of mean SRT for each patient, each extra millimeter in vestibular aqueduct size was associated with SRT increase of 15.6 dB (95% CI, 1.08 to 27.6 dB). Each extra year before first audiogram was associated with SRT decrease of 3.1 dB (95% CI, −7.4 to 0.53 dB). The R2 (multivariate coefficient of determination) for this model was 0.20, indicating that about 20% of the variance observed in mean SRT was explained by vestibular aqueduct size and age at first audiogram.
Word recognition scores data were available for 35 patients, with 177 measurements available for these 35 patients for a mean (range) of 5 (1-18) tests per patient. The model predicting WRS indicated that for each extra millimeter of VA midpoint width, there was a corresponding decrease of 21% in WRS (95% CI, −0.35% to −27.4%). For each year increase in age at first presentation, there was a corresponding increase of 1.6% in WRS (95% CI, −1.53% to 4.75%), though this was not statistically significant. For each year after first audiogram testing, there was a decrease of 1.7% in WRS (95% CI, −3.08% to −0.22%).
Figure 5 depicts WRS results for those patients with 2 or more test results across the range of years for all patients.
Figure 5. Word Recognition Score Test Results Compared With Age of Testing for 22 Patients.
Twenty-two patients with 2 or more hearing test results for speech reception threshold and only the ears randomly selected for numerical analysis are included. Each graph represents an individual patient and shows a regression line of the speech reception threshold compared with the age of the patient at testing; dots represent each test result. Orange lines represent patients with a vestibular midpoint width that is less than the median width (2.2 mm) among the patient population for this study.
The linear model of mean WRS for each patient revealed that each extra millimeter in vestibular aqueduct size was associated with a 19.0% decrease in WRS (95% CI, −38.4% to −0.6%) and each extra year before first audiogram test was associated with a 3.7% increase in WRS (95% CI, −3.5% to 10.5%). The R2 statistic (multivariate coefficient of determination) for this model was 0.23, indicating that about 23% of the variance in mean WRS was explained by vestibular aqueduct size and age at first audiogram.
Randomly-selected data included the ear with greatest vestibular aqueduct midpoint width (5.9 mm), an outlier that is depicted in mean audiometry vs vestibular aqueduct midpoint width (Figure 2 and Figure 3). When the outlying data point was removed from mixed-effects models, each extra millimeter of vestibular aqueduct midpoint width was associated with an increase of 14.2 dB in SRT (95% CI, 2.16 to 26.4 dB) and a decrease of 18.1% in WRS (95% CI, −33.3% to −3.2%). When the outlying data point was removed from linear models of mean values, each extra millimeter of vestibular aqueduct midpoint width was associated with an increase of 12.2 dB in SRT (95% CI, 1.1 to 26.3 dB) and a decrease of 14.2% in WRS (95% CI, −39.4% to 1.6%). The R2 statistic indicated that 20% of variance in SRT and 19% of the variance in WRS were explained by their respective models.
Discussion
Research examining the relationship between vestibular aqueduct size and hearing loss lacks consensus. Some studies have found no association between vestibular aqueduct size and hearing loss progression. In contrast, Antonelli et al found significant associations between vestibular aqueduct size and audiogram results. This ambiguity could be a result of study design issues such as limited sample size but could also be due to characteristics of EVA itself such as fluctuations in hearing loss. Resolving this ambiguity requires delineating between a baseline level of hearing loss and subsequent hearing loss progression. To this end, our study sought to separate the longitudinal hearing loss progression that may accompany EVA from initial hearing loss that may result from variable vestibular aqueduct size.
Our study improves understanding of EVA in several ways. First, by using repeated measures, we aimed to more accurately estimate hearing measurement trends. Repeated measures would increase accuracy because it is known that audiology measurement demonstrates variability in practice, and because hearing loss in EVA patients can fluctuate and progress. Compounding this issue, many previous studies of hearing measurement in patients with EVA have been cross-sectional or restricted to measurements at 2 points in time. In contrast, our study used a median of 5 SRT or WRS tests per patient, longitudinal depictions of which can be seen in Figure 4 and Figure 5.
Regarding audiology metrics, our study specifically focused on SRT and word recognition scores because these measures simultaneously evaluate speech discrimination and required sound intensity. Alternative hearing measurements include pure tones and pure tone average, which has been addressed by previous studies. Pure tone average offers a precise description of hearing loss because it is addresses single frequencies of sound, whereas SRT is the intensity in dBs at which a listener can repeat spondee (2-syllable words or sounds) at a rate of 50%, and WRS is the percentage of monosyllabic words correctly repeated at suprathreshold intensity. Speech reception threshold and pure tone average have high correlation, yet different contexts may favor the accuracy of one over the other for hearing measurement. We chose to use SRT and WRS to address perceptual and cognitive-linguistic factors, in contrast to measures of pure tones. This approach may be more relevant to patient care and communication, despite the strong correlations between PTA, SRT, and WRS.
Notably, we decided to include an outlying value in the final model (Figure 2 and Figure 3). The repeated-measures (mixed-effects) model is more appropriate for analysis of the available data, and thus is a better representation of effect estimates. While mixed-effects models that exclude the outlier remained significant, the relationship was less pronounced in models of mean values. Analysis that excludes the outlier, then, appears to support the same conclusions as those that include the outlier. Moreover, the outlying value of vestibular aqueduct midpoint width was 5.9 mm: this value may very well occur in practice, and was therefore considered informative for the final model.
Because there is no easily available alternative for mixed-effects models, one must defer to the linear model for a measure of variation explained by presented models. The R2 value explained about 20% of the variation in both our SRT and WRS models, indicating that a wide range of variation in hearing loss remains unaccounted for by our model.
Such variation may be the result of many different factors; however, one of the stronger candidates to explain this variation develops even before vestibular aqueduct size—that is, genetics. Given the significant variation in genotypes seen in patients with EVA, it is likely that there is some genetic component that may account for our unexplained variation in hearing loss. Unfortunately, the plethora of mutations demands multiple-comparisons approaches, such that an association difficult to verify. Zhao et al studied 66 mutations in 271 children and Okamato et al studied 4 mutations in 47 children, yet neither study detected statistically significant correlations between mutation and hearing. There is, then, a strong need for further research into the genetic associations between EVA and hearing loss. Whether a strength or weakness, our study demonstrates that there remain correlations to be drawn.
While other studies have mentioned vestibular aqueduct size, ours is one of few to address vestibular aqueduct size as a primary focus. Despite the parsimony of our model, if vestibular aqueduct size is itself determined by other factors such as patient genetics, it is possible that ours is a study examining an intermediate component or even byproduct of the causal pathway leading to hearing loss. Further research examining longitudinal hearing test results must be performed to verify an association between vestibular aqueduct size or mediating factors and hearing loss.
Our study also offers practical value by using a definition of enlarged vestibular aqueduct size according to a traditional measure, the Valavassori criterion. Valvassori et al defined an enlarged vestibular aqueduct as one that measures greater than 1.5 mm at its midpoint, yet this criterion has been demonstrated to be too stringent or considered too lax, depending on the study at hand. By following this traditional guideline, our results may be applied by clinicians with less suspicion of the applicability of the particular EVA definition used here.
Limitations
The study of patients with radiology-confirmed EVA means that our patient population represents those whose hearing merited further investigation by CT scan, thus it is likely that the proportion of EVA patients among candidates for inclusion in this work (14%) is an overestimate compared with the population at large. There may even be a separate population of patients with EVA whose hearing is below average yet whose EVA status is unknown and unexplored because of a lack of clinical significance.
Knowing that vestibular aqueduct size is related to hearing loss, and some degree of hearing loss progression may generally be expected, patients who have functional or better hearing should be closely followed and made aware of behaviors that may exacerbate hearing loss, including common life experiences like playing contact sports, swimming, or even flying. It is imperative, then, to continue research of patient experiences with sudden hearing loss progression and develop clear guidelines for EVA patients.
Conclusions
This longitudinal, repeated-measures study offers evidence that vestibular aqueduct size is directly related to hearing loss in patients with EVA. Using hundreds of speech reception threshold measurements, it was determined that each millimeter increase in vestibular aqueduct size is associated with an increase of 17.5 dB in speech reception threshold and a decrease of 21% in word discrimination tests. When consulting patients with EVA, these values may help clinicians set expectations with respect to vestibular aqueduct width and hearing loss.
References
- 1.Berrettini S, Forli F, Bogazzi F, et al. Large vestibular aqueduct syndrome: audiological, radiological, clinical, and genetic features. Am J Otolaryngol. 2005;26(6):363-371. [DOI] [PubMed] [Google Scholar]
- 2.Arcand P, Desrosiers M, Dubé J, Abela A. The large vestibular aqueduct syndrome and sensorineural hearing loss in the pediatric population. J Otolaryngol. 1991;20(4):247-250. [PubMed] [Google Scholar]
- 3.Alemi AS, Chan DK. Progressive hearing loss and head trauma in enlarged vestibular aqueduct: a systematic review and meta-analysis. Otolaryngol Head Neck Surg. 2015;153(4):512-517. [DOI] [PubMed] [Google Scholar]
- 4.Usami S, Abe S, Weston MD, Shinkawa H, Van Camp G, Kimberling WJ. Non-syndromic hearing loss associated with enlarged vestibular aqueduct is caused by PDS mutations. Hum Genet. 1999;104(2):188-192. [DOI] [PubMed] [Google Scholar]
- 5.Kemperman MH, Stinckens C, Kumar S, Huygen PL, Joosten FB, Cremers CW. Progressive fluctuant hearing loss, enlarged vestibular aqueduct, and cochlear hypoplasia in branchio-oto-renal syndrome. Otol Neurotol. 2001;22(5):637-643. [DOI] [PubMed] [Google Scholar]
- 6.Albert S, Blons H, Jonard L, et al. SLC26A4 gene is frequently involved in nonsyndromic hearing impairment with enlarged vestibular aqueduct in Caucasian populations. Eur J Hum Genet. 2006;14(6):773-779. [DOI] [PubMed] [Google Scholar]
- 7.Gopen Q, Zhou G, Whittemore K, Kenna M. Enlarged vestibular aqueduct: review of controversial aspects. Laryngoscope. 2011;121(9):1971-1978. [DOI] [PubMed] [Google Scholar]
- 8.Albert S, Blons H, Jonard L, et al. SLC26A4 gene is frequently involved in nonsyndromic hearing impairment with enlarged vestibular aqueduct in Caucasian populations. Eur J Hum Genet. 2006;14(6):773-779. [DOI] [PubMed] [Google Scholar]
- 9.Yang T, Gurrola JG II, Wu H, et al. Mutations of KCNJ10 together with mutations of SLC26A4 cause digenic nonsyndromic hearing loss associated with enlarged vestibular aqueduct syndrome. Am J Hum Genet. 2009;84(5):651-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Welling DB, Slater PW, Martyn MD, et al. Sensorineural hearing loss after occlusion of the enlarged vestibular aqueduct. Am J Otol. 1999;20(3):338-343. [PubMed] [Google Scholar]
- 11.Welling DB, Martyn MD, Miles BA, Oehler M, Schmalbrock P. Endolymphatic sac occlusion for the enlarged vestibular aqueduct syndrome. Am J Otol. 1998;19(2):145-151. [PubMed] [Google Scholar]
- 12.DeMarcantonio M, Choo DI. Radiographic evaluation of children with hearing loss. Otolaryngol Clin North Am. 2015;48(6):913-932. [DOI] [PubMed] [Google Scholar]
- 13.Deep NL, Hoxworth JM, Barrs DM. What is the best imaging modality for diagnosing a large vestibular aqueduct? Laryngoscope. 2016;126(2):302-303. [DOI] [PubMed] [Google Scholar]
- 14.Vijayasekaran S, Halsted MJ, Boston M, et al. When is the vestibular aqueduct enlarged? a statistical analysis of the normative distribution of vestibular aqueduct size. AJNR Am J Neuroradiol. 2007;28(6):1133-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valvassori GE, Clemis JD. The large vestibular aqueduct syndrome. Laryngoscope. 1978;88(5):723-728. [DOI] [PubMed] [Google Scholar]
- 16.Zalzal GH, Tomaski SM, Vezina LG, Bjornsti P, Grundfast KM. Enlarged vestibular aqueduct and sensorineural hearing loss in childhood. Arch Otolaryngol Head Neck Surg. 1995;121(1):23-28. [DOI] [PubMed] [Google Scholar]
- 17.Antonelli PJ, Nall AV, Lemmerling MM, Mancuso AA, Kubilis PS. Hearing loss with cochlear modiolar defects and large vestibular aqueducts. Am J Otol. 1998;19(3):306-312. [PubMed] [Google Scholar]
- 18.Zhao FF, Lan L, Wang DY, et al. Correlation analysis of genotypes, auditory function, and vestibular size in Chinese children with enlarged vestibular aqueduct syndrome. Acta Otolaryngol. 2013;133(12):1242-1249. [DOI] [PubMed] [Google Scholar]
- 19.Lai CC, Shiao AS. Chronological changes of hearing in pediatric patients with large vestibular aqueduct syndrome. Laryngoscope. 2004;114(5):832-838. [DOI] [PubMed] [Google Scholar]
- 20.Abou-Elew M, El-Khousht M, El-Minawi MS, Selim M, Kamel AI. Enlarged vestibular aqueduct in congenital non-syndromic sensorineural hearing loss in egypt. Indian J Otolaryngol Head Neck Surg. 2014;66(suppl 1):88-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Picard M, Banville R, Barbarosie T, Manolache M. Speech audiometry in noise-exposed workers: the SRT-PTA relationship revisited. Audiology. 1999;38(1):30-43. [DOI] [PubMed] [Google Scholar]
- 22.Hanekom T, Soer M, Pottas L. Comparison of the South African Spondaic and CID W-1 wordlists for measuring speech recognition threshold. S Afr J Commun Disord. 2015;62(1):E1-E10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saliba I, Gingras-Charland ME, St-Cyr K, Décarie JC. Coronal CT scan measurements and hearing evolution in enlarged vestibular aqueduct syndrome. Int J Pediatr Otorhinolaryngol. 2012;76(4):492-499. [DOI] [PubMed] [Google Scholar]
- 24.Mutai H, Suzuki N, Shimizu A, et al. Diverse spectrum of rare deafness genes underlies early-childhood hearing loss in Japanese patients: a cross-sectional, multi-center next-generation sequencing study. Orphanet J Rare Dis. 2013;8:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okamoto Y, Mutai H, Nakano A, et al. Subgroups of enlarged vestibular aqueduct in relation to SLC26A4 mutations and hearing loss. Laryngoscope. 2014;124(4):E134-E140. [DOI] [PubMed] [Google Scholar]
- 26.El-Badry MM, Osman NM, Mohamed HM, Rafaat FM. Evaluation of the radiological criteria to diagnose large vestibular aqueduct syndrome. Int J Pediatr Otorhinolaryngol. 2016;81:84-91. [DOI] [PubMed] [Google Scholar]
- 27.Moodie S, Rall E, Eiten L, et al. Pediatric audiology in North America: current clinical practice and how it relates to the American Academy of Audiology pediatric amplification guideline. J Am Acad Audiol. 2016;27(3):166-187. [DOI] [PubMed] [Google Scholar]