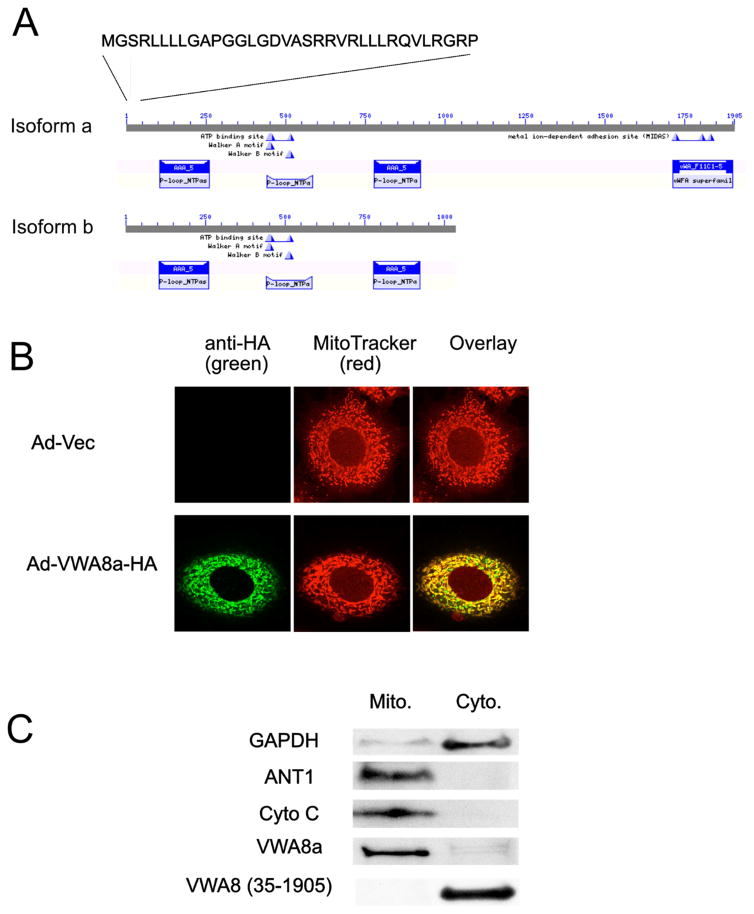
Figure 2. VWA8 has ATPase domains, a mitochondrial targeting sequence, and is a mitochondrial protein.
A. Domain architecture of VWA8a and VWA8b. Both isoforms have N-terminal mitochondrial targeting sequences and putative ATPase domains. B. Mouse AML12 hepatocytes were infected with adenoviral vectors encoding VWA8a with an HA tag or empty vector (control). Cells were stained with MitoTracker Red and a green fluorescent tagged anti-HA antibody. The overlap shows VWA8a is targeted to mitochondria. C. AML12 hepatocytes were fractionated into cytoplasmic and mitochondrial fractions and immunoblot analysis was used to detect endogenous VWA8a, GAPDH, adenine nucleotide translocase 1(ANT1) and Cytochrome C oxidase (Cyto C). Endogenous VWA8a was present only in the mitochondrial fraction. AML12 cells were transiently transfected with a vector encoding a VWA8a mutant, tagged with HA, and lacking the 1–34 N-terminal amino acid putative mitochondrial targeting sequence, VWA8 (35-1095). Immunoblot analysis with anti-HA antibody showed that this mutant was not targeted to mitochondria.