Figure 1.
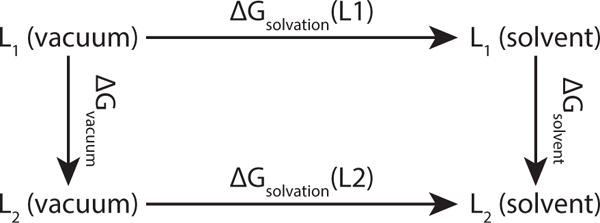
The relative solvation free energy ΔΔGsolvation (L1 → L2) between two ligands L1 and L2 is the difference between the horizontal (physical) processes, ΔGsolvation (L2) − ΔGsolvation (L1). These two free energies are typically difficult to calculate, but their difference is equal to the difference between the two vertical (alchemical) processes, ΔGsolvation − ΔGvacuum, which can each be readily calculated with free energy methods.
