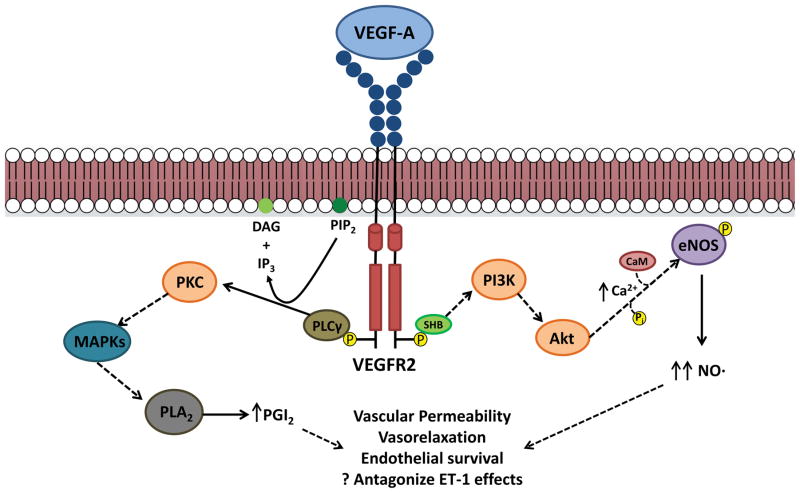
Figure 2. Intracellular signaling pathways for VEGFA/VEGFR2.
Upon ligand binding, VEGFR2 dimerizes and activates its receptor tyrosine kinase activity, which results in auto-phosphorylation of the intracellular domains. This can then lead to activation of a variety of signaling pathways. Intracellular calcium levels increase through activation of PI3K/Akt signaling, which activate endothelial nitric oxide synthase (eNOS) through calmodulin (CaM) binding as well as direct phosphorylation, resulting in increased nitric oxide (NO) production. VEGFR2 signaling also activates phospholipase C gamma (PLCγ), which converts phosphotidylinositol 4,5-bisphosphate (PIP2) to diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3). IP3 can also mediate increases in intracellular calcium, while DAG can activate protein kinase C (PKC). One downstream target of PKC signaling, through mitogen activated protein kinases (MAPKs), is phospholipase A2, ultimately leading to elevated levels of prostacyclin (PGI2). Both PGI2 and NO mediate many of the biological consequences of VEGF signaling, including enhanced vascular permeability, vasorelaxation, and endothelial survival. These may also antagonize the effects of vasoconstrictive mediators such as endothelin-1 (ET-1).