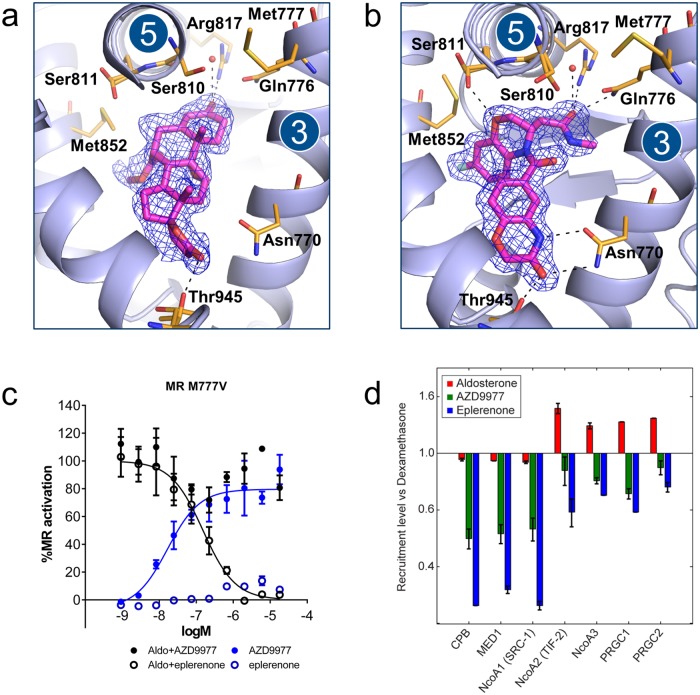
Fig 3. Crystal structure, reporter gene data on M777V mutation and co-peptide recruitment comparing AZD9977 and eplerenone.
The structures of the mineralocorticoid receptor (MR) ligand binding domain (LBD) in complex with (a) eplerenone and (b) AZD9977. The ligands are shown in magenta surrounded by refined 2mFo-DFc electron density as indicated by blue mesh. Putative hydrogen bonds are marked as dashed lines. Near the interface in between helices 3 and 5, the AZD9977 amide extension is placed with the oxygen in a position within hydrogen bonding distance of Gln776, Arg817, Ser810 and an ordered water molecule. In contrast, the eplerenone 3-keto group is further removed from the helix 3–5 interface and only appeared to make a direct interaction with Arg817. In addition, the AZD9977 3,4-dihydro-2H-1,4-benzoxazine oxygen is positioned to make a direct interaction to one of the observed rotamers of Ser811. As Ser811 is unique to MR within the steroid receptor family, this interaction may contribute to the selectivity profile of AZD9977. At the other end of the ligand binding pocket, the AZD9977 N-methyl-acetamide side chain is optimally placed to form a direct interaction to Thr945 and a bidentate interaction with Asn770. While the eplerenone γ-lactone is positioned to interact with Thr945 it is further away from Asn770 and only appears to make a weak interaction with the asparagine Nδ. (c) Reporter gene data for AZD9977 (solid) or eplerenone (open) tested on Met777Val MR mutation in presence (black) or absence (blue) of 0.1 nM aldosterone. n = 4, average ± SD. (d) Recruitment, relative to reference compound (dexamethasone), of MR-LBD to seven different co-regulator peptides for aldosterone (green), AZD9977 (red) and eplerenone (blue) at saturated binding conditions (50 μM). n = 2, average ± SD.