ABSTRACT
The host immune system generally serves as a barrier against tumor formation. Programmed death-ligand 1 (PD-L1) is a critical “don't find me” signal to the adaptive immune system, whereas CD47 transmits an anti-phagocytic signal, known as the “don't eat me” signal, to the innate immune system. These and similar immune checkpoints are often overexpressed on human tumors. Thus, dual targeting both innate and adaptive immune checkpoints would likely maximize anti-tumor therapeutic effect and elicit more durable responses. Herein, based on the variable region of atezolizumab and consensus variant 1 (CV1) monomer, we constructed a dual-targeting fusion protein targeting both CD47 and PD-L1 using “Knobs-into-holes” technology, denoted as IAB. It was effective in inducing phagocytosis of tumor cells, stimulating T-cell activation and mediating antibody-dependent cell-mediated cytotoxicity in vitro. No obvious sign of hematological toxicity was observed in mice administered IAB at a dose of 100 mg/kg, and IAB exhibited potent antitumor activity in an immune-competent mouse model of MC38. Additionally, the anti-tumor effect of IAB was impaired by anti-CD8 antibody or clodronate liposomes, which implied that both CD8+ T cells and macrophages were required for the anti-tumor efficacy of IAB and IAB plays an essential role in the engagement of innate and adaptive immune responses. Collectively, these results demonstrate the capacity of an elicited endogenous immune response against tumors and elucidate essential characteristics of synergistic innate and adaptive immune response, and indicate dual blockade of CD47 and PD-L1 by IAB may be a synergistic therapy that activates both innate and adaptive immune response against tumors.
KEYWORDS: adaptive immunity, CD47, dual-targeting fusion protein, innate immunity, immune checkpoint, PD-L1
Introduction
When the host immune system fails to block tumor formation, activation of the immune response could contribute to tumor regression.1 Immune checkpoint blockade therapies prove that an endogenous immune response can regress human tumors, but significant responses remain restricted to a minority of patients.2,3 PD-1 (Programmed death-1) expressed on effector T cells participate in peripheral tolerance and play a key role in immune suppression in cancer and chronic infectious diseases. When binding to its ligand PD-L1 which is highly expressed on the surface of tumor cells, PD-1 could send to the immune system a “don't find me” signal4 which inhibits T cell-mediated immune response and contributes to tumor immune evasion. Several anti-PD-1 or anti-PD-L1 monoclonal antibodies (mAbs) are currently providing evidence of clinical benefit in subsets of cancer patients.5 Atezolizumab (MPDL3280A), an anti-PD-L1 antibody, has shown promising results in patients with locally advanced or metastatic tumors.6 However, each treatment modality has limitations. Treatment withPD-1/PD-L1 blockade achieved about a 30% objective response rate (ORR) in several solid tumors, and the majority of patients are either resistant or relapse after a period of treatment, which highlights the need for the combination of anti-PD-1/L1 antibodies with other immune checkpoint antagonistic molecules or conventional therapies.2,7,8
Phagocytosis is an essential process utilized by an organism for pathogen or apoptotic cell clearance; however, increased expression of CD47 is proposed to be a mechanism through which cancer cells evade immune detection and phagocytosis.CD47, a widely expressed transmembrane protein, transmits a “don't eat me” signal to circulating macrophages via its receptor signal regulatory protein-α (SIRPα). SIRPαligation on macrophages and dendritic cells initiates a signaling cascade that culminates in the inhibition of phagocytosis of tumor cells.9,10 Human cancers broadly express CD47, and mRNA expression levels of CD47 have been shown to correlate with poor clinical outcomes in hematologic malignancies11,12 and most solid tumors.13 Anti-CD47 antibodies have been shown to promote phagocytosis in vitro and inhibit the growth of tumor, and even simultaneously induced an antitumor T-cell immune response in vivo.14 Accordingly, mAbs targeting CD4711,13or high-affinity SIPRα (CV1)15 were developed. Given the ubiquitous expression of the target, the “drug sink” represented by erythrocytes, platelets and other CD47-expressing cells may lead to a rapid elimination of anti-CD47 mAbs, and on-target toxicities such as anemia. To overcome these limitations, blockade of CD47-SIRPα interaction with single-chain variable fragments,16 CV1 monomer without Fc,15 Velcro-CD47,17 and bispecific antibodies targeting both CD47 and other tumor-related antigens18 were developed. CD47 has thus emerged as a promising target for cancer immunotherapy.
Although SIRPα-CD47 interaction may serve as a primary regulatory “don't eat me” signal on macrophages, recent work had demonstrated that PD-1 overexpression in mouse and human tumor-associated macrophages (TAMs) inhibited phagocytosis of cancer cell by TAMs.19,20 Furthermore, MYC oncogene was found to induce the expression of CD47 andPD-L1, and inactivation of MYC in mouse tumors down-regulated CD47 and PD-L1 expression and enhanced the antitumor immune response.4 CD47 and PD-L1 antagonistic molecules synergize to control B16F10 tumor growth and extend survival in vivo,21 and the combination of anti-human PD-L1 high-affinity consensus (HAC) and anti-CD47 antibody trended towards increasing survival in the DLD-1 xenograft model more than monotherapy.19 Together, these findings suggested that the combination of the CD47-targeting immunotherapy and anti-PD-L1 antibody may be essential in subsequent immunotherapy to boost the host's antitumor response by activation of macrophages and restoration of effector T cell functions.
Here, based on the variable region (VH and VL) of anti-PD-L1 antibody (atezolizumab) and CV1 monomer, we developed a dual-targeting fusion protein (denoted as IAB) using “Knobs-into-holes” technology. We studied IAB's ability to activate macrophages and T cells in vitro, and its antitumor effect in immune-competent mouse model of MC38. Our results show that both CD8+ T cells and macrophages were required for the anti-tumor efficacy of IAB. In conclusion, dual blockade of PD-L1 and CD47 by IAB represents a potential immunotherapeutic modality in cancer therapy.
Results
CD47 and PD-L1 were co-expressed on tumor cells
Solid tumors have been shown to evade host antitumor immunity through upregulation of the immune checkpoint PD-L1. CD47 has been found to be expressed on multiple human tumor types; up-regulation of CD47 expression in human cancers also allow tumors to evade the innate immune system's surveillance.9 As all human solid and blood tumor cells require CD47 expression to suppress phagocytic innate immune surveillance and elimination, CD47 is therefore a validated target for cancer therapies.13
We investigated the expression levels of CD47 and PD-L1 on the mouse solid tumor cells (4T1, MC38 and CT26) in vivo, which have been extensively used for preclinical assessment of anti-PD-1/L1 antibodies. Mouse tumor cell lines were inoculated subcutaneously into BALB/c or C57BL/6 mice, and on day 10, the tumors were removed and analyzed by flow cytometry. As shown in Fig. 1, both CD47 and PD-L1 were expressed on the tumor cells. Our previous results also demonstrated the co-expression of PD-L1 and CD47 in melanoma patients in clinic via immunohistochemistry (Fig. S1).Thus, we concluded that both CD47 and PD-L1 may also contribute to the tumor microenvironment through influence on macrophage phagocytosis and T-cell activation. Consequently, we speculated that targeting both innate and adaptive immune checkpoints (CD47 and PD-L1) by a dual-targeting fusion protein would likely maximize anti-tumor therapeutic effect and elicit more durable responses.
Figure 1.
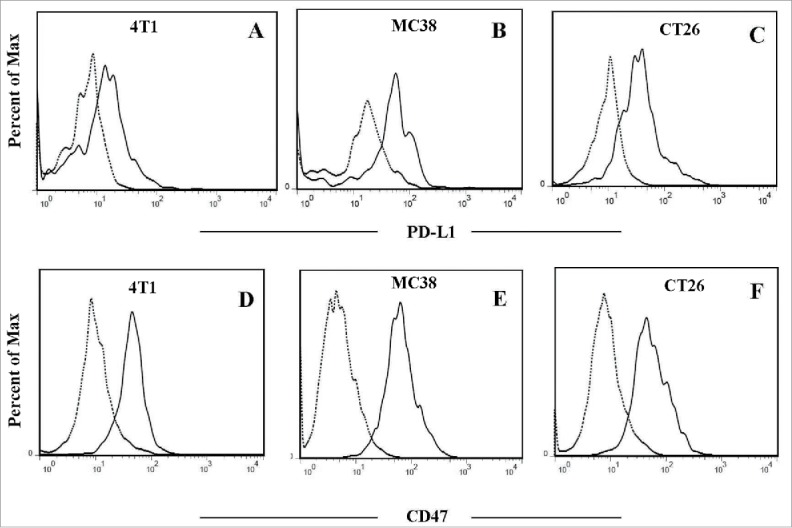
Co-expression of CD47 and PD-L1 on mouse tumor cells in vivo. Representative flow cytometry analysis following staining with anti-PD-L1 or anti-CD47 antibody (solid line) and isotype antibody as negative control (dotted line). A and D:4T1 tumor cells; B and E:MC38 tumor cells;C and F:CT26 tumor cells.
Construction, expression and characterization of IAB
Atezolizumab (TECENTRIQ®), a phage-derived human IgG1 antibody targeting PD-L1, has been shown to have anti-tumor activity in non-clinical assays and in clinical studies, and was first approved by the US Food and Drug Administration in May 2016.6,22 Relative to wild-type SIRPα, CV1 is an engineered high-affinity SIRPα variant, with affinity to CD47 ∼ 50,000-fold increased, which exhibited remarkable synergy with all tumor-specific mAbs tested by increasing phagocytosis in vitro and enhancing antitumor responses in vivo.15 In this study, we constructed an innate and adaptive immunity-dependent bispecific fusion protein(denoted as IAB) based on the variable region of atezolizumab and CV1 monomer in a IgG1 backbone using “Knobs-into-holes” technology (as shown in Fig. 2A).
Figure 2.
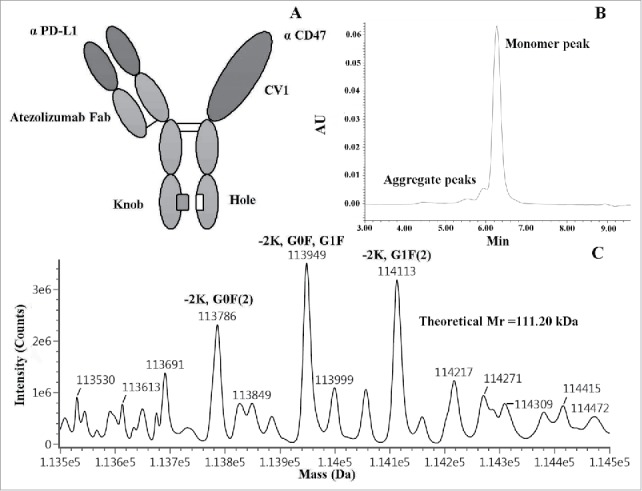
Design and characterization of IAB. (A) The schematic diagram of IAB. (B) SEC chromatogram (SEC-UPLC) of IAB. (C) MS analysis indicates the mass of main peaks were in good agreement with expected heterodimer with post-translational modifications. -2K,G0F(2): IAB-Lysine C-TERM(2),Glycosylation G0F(2), Expected mass: 113784Da, Mass error: 9.94ppm; -2K,G0F,G1F: IAB-Lysine C-TERM(2),Glycosylation G0F,Glycosylation G1F, Expected mass: 113946Da, Mass error: 26.88ppm; -2K,G1F(2): IAB-Lysine C-TERM(2),Glycosylation G1F(2), Expected mass: 114108Da, Mass error: 48.54Da.
The IAB was stably expressed by Chinese hamster ovary (CHO) cells. The final compound contained about 5% homodimers and aggregates, as measured by size-exclusion chromatography (SEC) (Fig. 2B), in line with other “Knobs-into-holes” antibodies produced by CHO cells.23,24 IAB was then characterized by non-reducing and reducing SDS-PAGE (Fig. S2), and was also analyzed at the intact protein level by liquid chromatography-mass spectrometry (LC-MS). As shown in Fig. 2C, the mass of main peaks were in good agreement with expected heterodimer with post-translational modifications.
To confirm the dual binding activity of IAB to each antigen, the CD47/PD-L1 binding activity of IAB was evaluated by competitive enzyme-linked immunosorbent assay (ELISA). As shown in Fig. 3A-B, the affinity of IAB to CD47 (IC50 = 2.897) was about ∼3-fold decreased compared to the avidity of CV1Fc (two CV1 domains) (IC50 = 0.918), while the affinity of IAB to PD-L1 (IC50 = 11.76) was about 10-fold decreased compared to the avidity of atezolizumab (IC50 = 0.948). Since IAB had one anti-PD-L1 antigen-binding fragment and one CV1 domain, it was deemed that IAB had lower affinity to each antigen than the parental clones, which was consistent with the previous report.25 Additionally, the binding activity of IAB with mouse tumor (MC38) cells and human tumor (HL60) cells were investigated by flow cytometry-based binding assay (Fig. S3).
Figure 3.
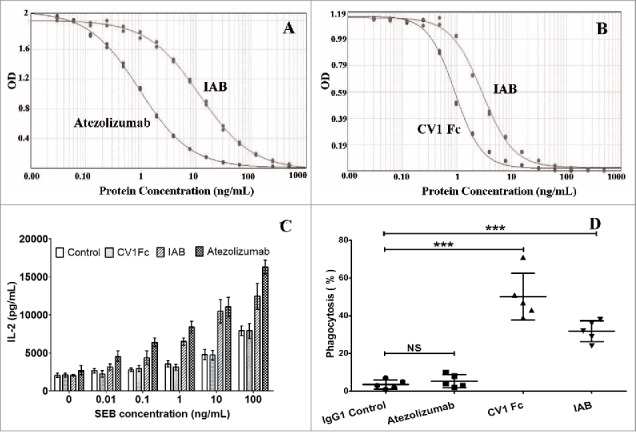
Competitive binding assay and in vitro human PBMC and murine macrophage activation. Competitive inhibition of IAB binding to PD-L1 (A) or CD47 (B) was evaluated by competitive ELISA assay (n = 2). (C) Dose increase in IL-2 secretion induced by IAB compared with CV1 and atezolizumab in SEB-stimulated PBMCs in a dose-dependent manner (n = 3). (D) IAB-mediated phagocytosis of MC38, CFSE-labeled MC38 cells were incubated with murine macrophages (RAW264.7) and indicated antibodies/proteins, the phagocytosis index was determined by flow cytometry (n = 5). Data are reported as the means ± SD, ***: p < 0.001; NS: non-significant (unpaired Student's t test).
These results demonstrate that IAB, aglycosylated heterodimeric Fc-fusion protein, was successfully prepared using the “Knobs-into-holes” platform.
In vitro human peripheral blood mononuclear cells and murine macrophage activation
Because IAB showed CD47/PD-L1 binding behavior that was similar to the parental proteins, we investigated whether the activation effects of IAB remained. Firstly, the stimulation of human peripheral blood mononuclear cells (PBMCs) with IAB and super-antigen Staphylococcal enterotoxin B (SEB) was conducted. As shown in Fig. 3C, PD-L1 blockade by atezolizumab or IAB significantly enhanced the SEB-stimulated PBMC's IL-2 secretion. Subsequently, we investigated whether IAB promotes phagocytosis of macrophages. Fluorescently labeled murine tumor cells MC38 and macrophages (RAW264.7) were co-cultured in the presence of IAB or parental proteins, and the phagocytic index was calculated as the percentage of phagocytosed CFSE+ cells. As shown in Fig. 3D, macrophages phagocytized MC38 tumor cells at a low frequency in the presence of atezolizumab (5.4%). However, CV1Fc (50.2%) and IAB (31.8%) significantly increased the phagocytosis of MC38. These results were consistent with our preliminary study that CV1 increased phagocytosis of tumor cells by mouse macrophage in vitro,26 and validated by mouse bone marrow-derived macrophages by immunofluorescence microscopy (Fig. S4). These data revealed that IAB was effective in inducing the phagocytosis by macrophages and stimulating T-cell activation in vitro.
IAB exhibits superior antibody-dependent cell-mediated cytotoxicity activity in vitro and minimal blood toxicity in vivo
To our knowledge, most of the anti-PD-1/PD-L1 antibodies in clinical development are either of the IgG4isotype (nivolumab,27 pembrolizumab28), which does not mediate antibody-dependent cell-mediated cytotoxicity (ADCC), or of FcγR-binding deficient IgG1 isotype (atezolizumab,6 MEDI473629), which is engineered to eliminate ADCC activity.30,31 However, a recent study has shown that avelumab (MSB0010718C) efficiently mediates ADCC of PD-L1-expressing tumor cells in vitro; only minor levels of lysis by ADCC were noted when unstimulated PBMCs were used as targets.32 Moreover, it was also proved that Fcγ receptor engagement augments the anti-tumor activity of anti-PD-L1 antibodies,30 which supported the use of anti-PD-L1 antibody with potent ADCC activity. As the wild-type IgG1 backbone was chosen for IAB in this study, the ADCC potential of IAB was also assessed in a reporter gene assay, which is equivalent to a lactate dehydrogenase ADCC bioassay in testing ADCC activity. As shown in Fig. 4A, IAB was more potent than the parental anti-PD-L1 antibody at inducing ADCC in low concentration. Furthermore, the ADCC assay showed IAB was capable of activating ADCC luciferase reporter signaling in a markedly dose-dependent manner that is similar to CV1 Fc. Atezolizumab with IgG1mutation backbone (N297A) did not induce ADCC in vitro, which was consistent with results previously reported.6 Additionally, it was assumed that the lower affinity of IAB to MC38 cells compared toCV1 Fc may result in the lower ADCC activity induced by IAB compared to CV1 Fc.
Figure 4.
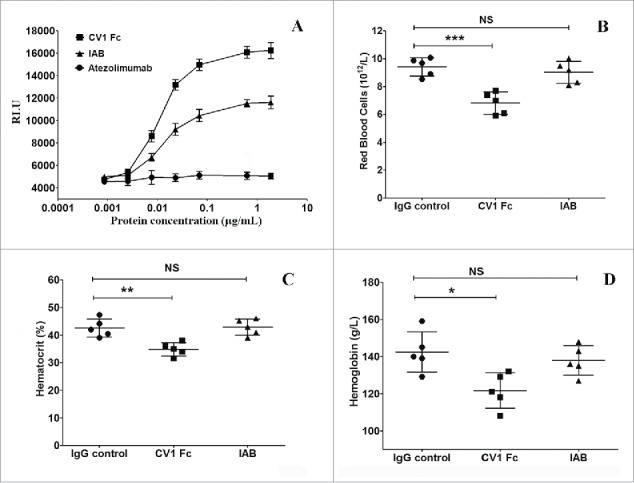
IAB exhibits superior ADCC activity in vitro and minimal blood toxicity in vivo. (A) Relative ADCC activity of IAB, CV1 Fc and atezolizumab in ADCC reporter assay. (n = 3); Analysis of red blood cell (B), hematocrit (C) and hemoglobin (D) in mice intraperitoneal injected with 100 mg/Kg of IAB or parental proteins (n = 5). Data are reported as the means ± SD, *: p<0.05, **: p <0.01, ***: p <0.001; NS: non-significant (unpaired Student's t test).
Due to the widespread expression of CD47 on tumor cells and normal cells, these strategies targeting CD47 (anti-CD47 antibodies or CV1 Fc) are handicapped by large antigen sinks in vivo and indiscriminate cell binding. Even more, these factors reduce bioavailability of anti-CD47 mAbs and increase the risk of blood toxicity in vivo. PD-L1 shows a broad expression profile from tumor cells to immune cells, particularly high in immunoprivileged sites of the body. Its relative expression could be higher in tumor tissues. Thus, tumor-specific delivery of CD47 antagonist would generate better anti-tumor effect with fewer side effects than systemic administration. To evaluate the hematological toxicity of IAB in vivo, blood samples of healthy BALB/c mice treated with high doses of IAB or parent protein (100 mg/Kg) were analyzed. As illustrated in Fig. 4 B-D, no obvious sign of hematological toxicity was observed for IAB injected into mice at the dose of 100 mg/kg, while red blood cells, hematocrit and hemoglobin were dramatically reduced for CV1 Fc injected mice. Our results were also in agreement with those reported for other CD47 antagonistic molecules in dual-targeting bispecific format.18,33
Both innate and adaptive immunity are essential for IAB-mediated tumor regression
Previously, it was reported that CD47 antagonistic molecules synergizes with other tumor-specific antibodies to promote macrophage-mediated tumor eradication across a range of xenogeneic mouse models of human cancer.21 However, the use of imunocompromised hosts (T cell-, NK cell-, and B-cell-deficient) has precluded assessment of a role for adaptive immunity. To explore the synergistic anti-tumor effects of IAB in vivo, an immunocompetent host harboring a syngeneic tumor (MC38) was used in our study. After MC38 tumor cells were implanted subcutaneously on the flank of C57BL/6mice, IAB, indicated antibodies (10 mg/Kg) or control IgG were administered by intra-tumor injection (on days 7, 10), which could avoid the “antigen sink” induced by normal CD47-expressing cells. As shown in Fig. 5A, both CV1Fc and atezolizumab were effective in delaying the MC38 tumor progression (824±113mm3or280± 61mm3). It is particularly noteworthy that treatment with IAB resulted in rapid and nearly complete tumor regression (58±37 mm3). These results indicate that IAB exhibited potent antitumor activity in immune-competent mouse model, and IAB was able to recapitulate the synergistic effect of anti-CD47 and anti-PD-L1.
Figure 5.
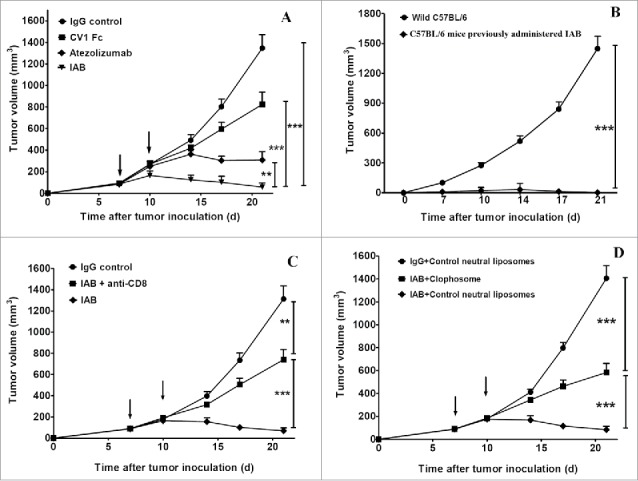
The durable anti-tumor effect of IAB depends on both innate and adaptive immunity. (A) Anti-tumor effect: C57BL/6 (n = 5/group) mice were injected s.c. with 2 × 105 MC38 cells and treated intratumorally with indicated antibodies at the dose of 10mg/Kg on day 7, and 10. (B) The re-challenge experiment: C57BL/6 mice previously administered with IAB or wild C57BL/6 (n = 5/group) mice were challenged s.c. with 1 × 106 MC38 cells. (C) The CD8+ depletion experiment: MC38 tumor bearing C57BL/6 (n = 5/group) were treated intratumorally with IAB (10mg/Kg) and injected i.p.with 400 μg anti-CD8 antibody on days 7, and 10. (D) The macrophage depletion experiment: MC38 tumor bearing C57BL/6 (n = 5/group) were treated intratumorally with IAB (10mg/Kg) on days 7, and 10. 200 μl of clophosome or control liposome was administered i.p. on day 5 and every 4 days thereafter. Data are reported as means ± SEM. *: p < 0.05, **: p < 0.01,***: p < 0.001 (unpaired Student's t test).Note: For negative controls shown in Figure 5C (IgG control group) and 5D (IgG+Control neutral liposomes group), mice bearing too large or small tumor after MC38 inoculation were excluded; on day 7 after tumor inoculation, mice with measurable tumor (~100 mm3) were randomized to treatment group. The error bars represent standard error of the mean (SEM). In Figure 5C, the IgG control group mean±SEM on Days 7, 10, 14, 17 and 21 were 89±10, 185±31, 399±51, 726±87, 1297±130, respectively. In Figure 5D, the IgG control neutral liposomes group mean±SEM on Days 7, 10, 14, 17 and 21 were 92±12, 193±20, 410±35, 800±55, 1403±122, respectively.
To investigate whether the immune response activated by IAB could elicit durable anti-tumor immunity, mice that survived after an initial course of IAB treatment were re-challenged with MC38 cells (1 × 106 cells) in the contralateral flank. As shown in Fig. 5B, all mice surviving a primary challenge rejected a secondary challenge with MC38. The results demonstrate that IAB treatment can elicit durable systemic immune memory to prevent relapse, which also was consistent with previous observations that targeting both CD47 and PD-L1 can potentiate the vaccinal effect of antitumor response.21,34
To test the essential role of innate and adaptive immune system for IAB-mediated tumor control in mouse tumors, CD8+T-cell-depleted or macrophage-depleted C57BL/6 mice tumor models were generated using anti-CD8 antibody or liposomal clodronate. In the absence of CD8+ T cells or macrophages, the therapeutic effect of IAB was dramatically abrogated (shown in Fig. 5 C-D), which implied that both CD8+ T cell and macrophage were required for the anti-tumor efficacy of IAB and IAB plays an essential role to engage innate and adaptive immune responses.
Discussion
Harnessing the immune system against cancer is becoming an increasingly effective therapy option that can result in dramatic and durable responses in several cancer types.35 The PD-1/PD-L1 pathway has a pivotal role in dampening immunosurveillance of tumors, and therapy directed to these two targets could have durable antitumor effects and tolerable immune-related toxicity. Nevertheless, it is important to recall that at least 70% of cancer patients have not responded very well to anti-PD-1 antibody treatment,36 which suggests that PD-1 blockade as a monotherapy may be successful in the setting of a pre-existing antitumor immune response in the patient.37 Recent clinical or preclinical studies suggested that anti-PD-L1 antibodies in combination with antagonists of other checkpoints, such as CTLA-4,38 LAG-3,39 can produce additive or synergistic antitumor immunity. In our study, dual-blockade of CD47 and PD-L1 was applied to boost host innate and adaptive immunity, based on the findings that cancer cells in the tumor microenvironment can simultaneously up-regulate CD47 and PD-L1.
Bispecific antibodies (BsAbs) represent an emerging class of antibody therapeutics that exhibit specific binding to 2 different antigens. Many formats of BsAbs have been engineered using recombinant approaches, including IgG-like BsAbs, dual-variable domain (DVD) molecules, and bispecific T-cell engagers (BiTE).40 Although the BiTE molecule blinatumomab (BLINCYTO®) is approved in the US and European Union, bispecific antibody or fusion protein with near-native antibody architecture using “Knobs-into-holes” technology demonstrate pharmacokinetic properties more similar to the conventional antibody.41 We thus constructed the IAB using “Knobs-into-holes” technology. Because of the abundant expression of CD47 in healthy cells, the efficacy and the safety of these CD47 blocking strategies may be negatively affected by antigen sink, which would reduce the bioavailability and increase the risk of hematologic toxicity in vivo. The advantage of the dual-targeting fusion protein format was that tumor-specific delivery of aCD47 antagonist would generate better anti-tumor effects with fewer side effects than systemic administration. For example, a bispecific DVD-Ig (Dual-Variable Domain Immunoglobulin) antibody targeting CD47 and CD20 selectively binds and eliminates dual antigen expressing lymphoma cells, but binds poorly to human red blood cells.33 Similarly, IAB was capable of inducing ADCC in vitro, but no obvious sign of hematological toxicity was observed in vivo, even at a high dose (100 mg/Kg).
CD47 blockade enhances the engulfment of tumor cells by macrophages, and enables dendritic cells to process and present tumor antigens as shown by previously published findings.14,34 This can lead to the priming and boosting of tumor-specific CD8+ effector T cells,14,34 raising the possibility of combining CD47-targeted therapies with T-cell checkpoint blockade to unleash both an innate and adaptive antitumor response. Using syngeneic immunocompetent mouse model of MC38, we revealed that IAB could recapitulate the therapeutic synergy of anti-CD47 and anti-PD-L1 combination therapy. Moreover, treatment of mouse bearing tumors with IAB could elicit durable systemic immune memory to prevent relapse, and potentiate the vaccinal effect of antitumor response. Finally, we showed that both macrophage and CD8+ T cell were required for the anti-tumor efficacy of IAB, and IAB plays an essential role to engage innate and adaptive immune responses.
Recently, it has been shown that the therapeutic effect of anti-HER2/neu antibody42 or cetuximab43 depends on both NK cells and T cells. In addition, the combinations of antibody and interferon-beta therapy targeting the tumor microenvironment could bridge innate and adaptive immune responses, which is more potent than the first generation of antibodies for controlling antibody-resistant tumors.44 It was also reported that Fc/IL-2 synergized with the antibody TA99 to control B16F10 growth by inducing both innate and adaptive immune response.45 Collectively, the strategies used in this study open avenues for synergistic innate and adaptive immune response against tumors, which may have great impact in antitumor drug discovery. IAB targeting PD-L1 and CD47 was also shown to be a potential drug candidate for cancer treatment.
Materials and methods
Cell Lines, animals and other reagents
Murine colon adenocarcinoma cell lineMC38 was obtained from State Key Laboratory of Antibody Medicine and Targeted Therapy (Shanghai, China). The murine metastatic colon carcinoma cell line CT26 (CRL-2638), mouse breast tumor cell line 4T1(CRL-2539) and murine macrophages RAW264.7 (TIB-71™), human promyelocytic leukemia cells HL 60 (CCL-240) were obtained from American Type Culture Collection.
CV1 Fc with IgG1 backbone and atezolizumab were produced by Shanghai Sinomab Biotechnology Co (Shanghai, China), as described previously.15 FITC-conjugated goat anti-human IgG (62-8411) was purchased from Thermo Fisher. Anti-CD8 depleting antibody (cloneLyt2) was produced in-house. Six-8-wk-old wild-type female BALB/c or C57BL/6 mice were purchased from Shanghai Laboratory Animals Center (Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences; Shanghai, China).
Mice were euthanized with CO2 asphyxiation. All animals were treated in accordance with guidelines of the Committee on Animals of the Shanghai Research Centre for Model Organisms. All experiments were conducted in accordance with institutional guidelines and under an Institutional Animal Care and Use Committee (IACUC) protocol.
PD-L1 and CD47 co-expression analysis
Tumor cells (5 × 105) were inoculated subcutaneously into the shaved left back of 6–8-wk-old BALB/c or C57BL/6 mice. On day 10, solid tumors (>800 mm3) were excised, ground to prepare cell suspensions and digested with Collagenase IV (Invitrogen, 17104–019) and DNase I (Stemcell, 07900),Then, the single-cell suspensions subsequently incubated with atezolizumab or CV1 Fc and isotype control (CMAB801, Shanghai Sinomab Biotechnology Co.). FITC-conjugated goat anti-human IgG (Thermo Fisher) was used as the secondary antibody, further flow cytometric analysis was performed on a BD FACSCalibur system (BD Biosciences). All downstream analysis was performed in FlowJo (Treestar Inc.).
Bispecific fusion protein expression and purification
The light chain κ and heavy chain of human IgG1 subclass were chosen as the basic framework for IAB using “Knobs-into-holes” technology; the amino sequence of IAB was provided in Supplementary material (Appendix 1, 2, 3). The variable region of atezolizumab was fused to the “Knobs” (T366W) arm, andCV1was fused to the “Holes” (T366S, L368A, Y407V) arm (Fc CH2 CH3). The light chain, the heavy chain (Knobs) and the CV1-Fc (Holes) chain mentioned above were each cloned into a pcDNA3.1 (+) vector. Then, the expression vectors were co-transfected into CHO-K1 cells. IAB was subsequently expressed and purified according to previous methods.23,46
Characterization of IAB by SEC-UPLC, LC-MS, and competitive ELISA
The homogeneity of IAB were verified by size-exclusion chromatography-ultra performance liquid chromatography (SEC-UPLC) and molecular weight was accurately measured by LC-MS, as previously described.47,48
The CD47/PD-L1 binding activity of IAB was evaluated by competitive ELISA. Briefly, ELISA plates (Costar) were coated overnight at 4°Cwith hPD-L1 Fc (Shanghai Sinomab Biotech Co., ZJ-01-041) or hCD47 Fc (Shanghai Sinomab Biotech Co., ZJ-01-053) in phosphate-buffered saline (PBS) buffer. Followed by blocking with 10% nonfat dried milk (Bright Dairy, China). Indicated concentrations of competing IAB (0.02∼1000 ng/mL) were applied to the ELISA plate containing biotin-conjugated atezolizumab or CV1 Fc (Shanghai Sinomab Biotechnology Co., ZJ-01-055), and incubated for 2 h at 37°C. After washing with phosphate-buffered saline with Tween 20 (PBST, in house), avidin-HRP (eBioscience) was added and incubated at 37°C for1 h, detection was performed by the addition of 3,3′,5,5′–tetramethylbenzidine (TMB) substrate reagent for 15 minutes followed by 2 M hydrochloric acid. Absorbance at 450 nm was measured by SpectraMax M5 Multimode Plate Reader (Molecular Devices) and data were fitted using a sigmoidal 4-parameter curve by SoftMaster (Honeywell).
In vitro human PBMC and murine macrophage activation
PBMC (1 × 105/well) from independent healthy donors (N = 3) by using a monocyte purification kit (Miltenyi Biotec) were cultured for 3 days at 37°C with RPMI 1640 medium, indicated antibody (IAB, Atezolizumab, CV1 Fc) and isotype control (CMAB801, Shanghai Sinomab Biotechnology Co.) were added at 20 μg/mL in 96-well plate (Corning), serial dilutions of staphylococcal enterotoxin B (SEB; Shanghai Sinomab Biotech Co., ZJ-01-051) were added at the initiation of the assay. After 3 days, the IL-2 levels in culture supernatants were measured using a Human IL-2 ELISA Kit (eBioscience), as previously described.27
For the in vitro phagocytosis assay, 5 × 104cells/well murine macrophages (RAW264.7) were plated with 2 × 105MC38 cells labeled by 0.5 µM carboxyfluoresceindiacetatesuccinimidyl ester (CSFE) in serum-free RPMI medium in a 96-well ultra-low adherent plate (Corning). The 10 μg/mL indicated antibodies (atezolizumab, CV1Fc or IAB) or isotype control (CMAB801, Shanghai Sinomab Biotechnology Co.) were added and incubated for 4h at 37°C. Then cells were washed twice with PBS, macrophages were stained with anti-mouse F4/80-PE antibody (eBioscience, 12-4801-80) for FACSCanto II analysis (BD Biosciences). The phagocytic index was calculated as the percentage of phagocytosed CFSE+ cells, as previously described.14
ADCC of IAB in vitro
ADCC-reporter gene bioassay was performed according to previous report.46,49 MC38 cells, which overexpressed PD-L1 and CD47, were seeded at 1 × 104/ well in an opaque 96-well tissue culture plate. IAB, CV1 Fc or atezolizumab were serially diluted and incubated with the MC38 cells for approximately 1 h at 37°C, 5% CO2. Following incubation, Jurkat-expressing FcγRIIIa luciferase reporter cells (Shanghai Sinomab Biotech Co) (5 × 104/ well) were added to the MC38/antibody mixture per well. The mixture was incubated for approximately 4 h at 37°C, 5% CO2, and then measured for luciferase production using a luminescent substrate (Promega Bright Glo).
Hematologic toxicity of IAB in vivo
Healthy 6–8-wk-old BALB/c mice were injected intraperitoneally (i.p.) with the indicated antibodies (atezolizumab, CV1Fc or IAB) or isotype control (CMAB801, Shanghai Sinomab Biotechnology Co.) at a dose of 100 mg/Kg (n = 5).The next day, blood was drawn from the retro-orbital plexus and collected in dipotassium-EDTA tubes (BD Biosciences), and analyzed by UniCel DxH800 Coulter Cellular Analysis (Beckman Coulter, Inc.) in Shanghai Biomodel Organism Science and Technology Development Co.
Anti-tumor effects of IAB and CD8 T cell or macrophage depletion in immunocompetent C57BL/6 mice
The immunocompetent mouse model was established in female 6–8-wk-old C57BL/6 by subcutaneous implantation of 2 × 105 MC38 cells. On day 7 after tumor inoculation, mice with measurable tumor (∼100 mm3) were randomized to treatment groups (n = 5). Indicated antibodies (atezolizumab, CV1Fc or IAB) or negative control (CMAB801, Shanghai Sinomab Biotechnology Co.) at doses of 10 mg/Kg were administered intratumorally on days 7, and 10. In the tumor-rechallenge experiment, mice that survived after an initial course of IAB treatment were re-challenged with MC38 cells (1 × 106 cells) in the contralateral flank. Tumor size was calculated as length × width2 × 0.5 mm3.
The CD8+ T cell or macrophage depletion in immunocompetent C57BL/6 mice (n = 5) was accomplished using anti-CD8 antibody or liposomal clodronate (FormuMax Scientific) i.p., respectively, as previously described.12,34 In CD8+ depletion experiments, 400 μg anti-CD8 antibody (clone Lyt2) was injected i.p. at the same time of the treatment of IAB. In the macrophage depletion experiments, 200 μl of clophosome or control liposomes was administered i.p. 2 days before of the treatment, and 100–200 μl of clophosome or control liposomes was then injected every 4 days in the periods of the treatment.
Mice were euthanized with CO2 asphyxiation. All experiments were conducted in accordance with institutional guidelines and under an IACUC protocol.
Statistical analysis
All data are presented as means ± SD or SEM. Statistical analysis and graph preparation were performed using Prism 6 (GraphPad Software). p values were calculated using unpaired parametric Student's t test, assuming equal variances. Statistically significant differences are marked as *p < 0.05, **p < 0.005,***p < 0.0001.
Supplementary Material
Disclosure of potential conflicts of interest
YJ Guo, WZ Qian, HZ Guo, J Xu are co-inventors of Chinese patent No. CN2016103729544 entitled “A dual-targeting fusion protein targeting CD47 and PD-L1”.
Acknowledgments
We are grateful for advice from Professor Yangxin Fu and Yang Pu, Department of Pathology and Committee on Immunology, University of Chicago. We thank Yimei Wu, Huiyong Zhang from Shanghai Sinomab Biotechnology Co., Ltd. for IAB protein preparation, and Xiaolian Yang, RuoyuXufrom Shanghai Sinomab Biotechnology Co., Ltd. for assistance with animal experiments. This work was supported by grants from National Science and Technology Major Projects for “Major New Drugs Innovation and Development”(2015ZX09101038); Shanghai Rising-Star Program (16QB1404300); Shanghai Key Technologies R&D Program (15431906100, 16431901200, 16431904700, 16142201700, 16430730400, 16DZ1910400); Shanghai Antibody Drug Development Public Service Platform (16DZ2292900).
Abbreviations
- ADCC
Antibody-dependent cell-mediated cytotoxicity
- BiTE
Bispecific T cell engager
- BsAbs
Bispecific antibodies
- CSFE
Carboxyfluoresceinsuccinimidyl ester
- CV1
Consensus Variant 1
- DVD
Double-variable domain
- DVD-Ig
Dual-Variable Domain Immunoglobulin
- HAC
High-affinity consensus
- IAB
Innate and adaptive immunity-dependent bispecific fusion protein
- LC-MS
Liquid chromatography-mass spectrometry
- mAbs
Monoclonal antibodies
- ORR
Objective response rate
- PBS
Phosphate-buffered saline
- PBST
Phosphate-buffered saline with Tween 20
- PD-1
(Programmed Death-1)
- SEB
Staphylococcal enterotoxin B
- SEC
Size-exclusion chromatography
- TAMs
Tumor-associated macrophages
- UPLC
Ultra Performance Liquid Chromatography
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–61. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moynihan KD, Opel CF, Szeto GL, Tzeng A, Zhu EF, Engreitz JM, Williams RT, Rakhra K, Zhang MH, Rothschilds AM, et al.. Eradication of large established tumors in mice by combination immunotherapy that engages innate and adaptive immune responses. Nat Med. 2016;22:1402–10. doi: 10.1038/nm.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casey SC, Tong L, Li Y, Do R, Walz S, Fitzgerald KN, Gouw AM, Baylot V, Gütgemann I, Eilers M, et al.. MYC regulates the antitumor immune response through CD47 and PD-L1. Science. 2016;352:227–31. doi: 10.1126/science.aac9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest. 2015;125:3384–91. doi: 10.1172/JCI80011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDermott DF, Sosman JA, Sznol M, Massard C, Gordon MS, Hamid O, Powderly JD, Infante JR, Fassò M, Wang YV, et al.. Atezolizumab, an anti-programmed death-ligand 1 antibody, in metastatic renal cell carcinoma: Long-term safety, clinical activity, and immune correlates from a phase Ia study. J Clin Oncol. 2016;34:833–42. doi: 10.1200/JCO.2015.63.7421. [DOI] [PubMed] [Google Scholar]
- 7.Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161:205–14. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen X, Xu J, Guo Q, Wang L, Yang Y, Guo H, Gu N, Zhang D, Qian W, Hou S, et al.. Therapeutic efficacy of an anti-PD-L1 antibody based immunocytokine in a metastatic mouse model of colorectal cancer. Biochem Biophys Res Commun. 2016;480:160–5. doi: 10.1016/j.bbrc.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 9.Chao MP, Weissman IL, Majeti R. The CD47-SIRPalpha pathway in cancer immune evasion and potential therapeutic implications. Curr Opin Immunol. 2012;24:225–32. doi: 10.1016/j.coi.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chester C, Marabelle A, Houot R, Kohrt HE. Dual antibody therapy to harness the innate anti-tumor immune response to enhance antibody targeting of tumors. Curr Opin Immunol. 2015;33:1–8. doi: 10.1016/j.coi.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 11.Kim D, Wang J, Willingham SB, Martin R, Wernig G, Weissman IL. Anti-CD47 antibodies promote phagocytosis and inhibit the growth of human myeloma cells. Leukemia. 2012;26:2538–45. doi: 10.1038/leu.2012.141. [DOI] [PubMed] [Google Scholar]
- 12.Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD Jr, van Rooijen N, Weissman IL. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138:286–99. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willingham SB, Volkmer JP, Gentles AJ, Sahoo D, Dalerba P, Mitra SS, Cohen JD, Lovelace P, Scheeren FA, Chao MP, et al.. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci U S A. 2012;109:6662–7. doi: 10.1073/pnas.1121623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tseng D, Volkmer JP, Willingham SB, Contreras-Trujillo H, Fathman JW, Fernhoff NB, Seita J, Inlay MA, Weiskopf K, Miyanishi M, Weissman IL. Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proc Natl Acad Sci U S A. 2013;110:11103–8. doi: 10.1073/pnas.1305569110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiskopf K, Ring AM, Ho CC, Volkmer JP, Levin AM, Volkmer AK, Ozkan E, Fernhoff NB, van de Rijn M, Weissman IL. Engineered SIRPalpha variants as immunotherapeutic adjuvants to anticancer antibodies. Science. 2013;341:88–91. doi: 10.1126/science.1238856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sagawa M, Shimizu T, Fukushima N, Kinoshita Y, Ohizumi I, Uno S, Kikuchi Y, Ikeda Y, Yamada-Okabe H, Kizaki M. A new disulfide-linked dimer of a single-chain antibody fragment against human CD47 induces apoptosis in lymphoid malignant cells via the hypoxia inducible factor-1alpha pathway. Cancer Sci. 2011;102:1208–15. doi: 10.1111/j.1349-7006.2011.01925.x. [DOI] [PubMed] [Google Scholar]
- 17.Ho CC, Guo N, Sockolosky JT, Ring AM, Weiskopf K, Ozkan E, Mori Y, Weissman IL, Garcia KC “Velcro” engineering of high affinity CD47 ectodomain as signal regulatory protein alpha (SIRPalpha) antagonists that enhance antibody-dependent cellular phagocytosis. J Biol Chem. 2015;290:12650–63. doi: 10.1074/jbc.M115.648220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masternak K, Johnson Z, Buatois V, Rousseau F, Magistrelli G, Moine V, et al.. Abstract B54: Antagonizing CD47-SIRP alpha interaction with a bispecific antibody: A novel cancer immunotherapy approach. Cancer Immunol Res. 2015;3:B54–B. doi: 10.1158/2326-6074.TUMIMM14-B54. [DOI] [Google Scholar]
- 19.Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN, Gupta R, Tsai JM, Sinha R, Corey D, et al.. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545:495–9. doi: 10.1038/nature22396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arlauckas SP, Garris CS, Kohler RH, Kitaoka M, Cuccarese MF, Yang KS, Miller MA, Carlson JC, Freeman GJ, Anthony RM, et al.. In vivo imaging reveals a tumor-associated macrophage-mediated resistance pathway in anti-PD-1 therapy. Sci Transl Med. 2017;9:eaal3604. doi: 10.1126/scitranslmed.aal3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sockolosky JT, Dougan M, Ingram JR, Ho CC, Kauke MJ, Almo SC, Ploegh HL, Garcia KC. Durable antitumor responses to CD47 blockade require adaptive immune stimulation. Proc Natl Acad Sci U S A. 2016;113:E2646–54. doi: 10.1073/pnas.1604268113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weinstock C, Khozin S, Suzman D, Zhang L, Tang S, Wahby S, Goldberg KB, Kim G, Pazdur R. U.S. food and drug administration approval summary: Atezolizumab for metastatic non-small cell lung cancer. Clin Cancer Res. 2017;23:4534–9. doi: 10.1158/1078-0432.CCR-17-0540. [DOI] [PubMed] [Google Scholar]
- 23.Ridgway JB, Presta LG, Carter P. ‘Knobs-into-holes’ engineering of antibody CH3 domains for heavy chain heterodimerization. Protein Eng. 1996;9:617–21. doi: 10.1093/protein/9.7.617. [DOI] [PubMed] [Google Scholar]
- 24.Shatz W, Chung S, Li B, Marshall B, Tejada M, Phung W, Sandoval W, Kelley RF, Scheer JM. Knobs-into-holes antibody production in mammalian cell lines reveals that asymmetric afucosylation is sufficient for full antibody-dependent cellular cytotoxicity. MAbs. 2013;5:872–81. doi: 10.4161/mabs.26307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milenic DE, Yokota T, Filpula DR, Finkelman MA, Dodd SW, Wood JF, Whitlow M, Snoy P, Schlom J. Construction, binding properties, metabolism, and tumor targeting of a single-chain Fv derived from the pancarcinoma monoclonal antibody CC49. Cancer Res. 1991;51:6363–71. [PubMed] [Google Scholar]
- 26.Zhao Y, Xu J, Guo H, Li Y, L J, Chen X, et al.. Construction and characterization of pro-SIRPα fusion protein. Curr Immunol. 2016;36:89–95. [Google Scholar]
- 27.Wang C, Thudium KB, Han M, Wang XT, Huang H, Feingersh D, Garcia C, Wu Y, Kuhne M, Srinivasan M, et al.. In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates. Cancer Immunol Res. 2014;2:846–56. doi: 10.1158/2326-6066.CIR-14-0040. [DOI] [PubMed] [Google Scholar]
- 28.Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al.. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–50. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 29.Stewart R, Morrow M, Hammond SA, Mulgrew K, Marcus D, Poon E, Watkins A, Mullins S, Chodorge M, Andrews J, et al.. Identification and characterization of MEDI4736, an antagonistic Anti-PD-L1 monoclonal antibody. Cancer Immunol Res. 2015;3:1052–62. doi: 10.1158/2326-6066.CIR-14-0191. [DOI] [PubMed] [Google Scholar]
- 30.Dahan R, Sega E, Engelhardt J, Selby M, Korman AJ, Ravetch JV. FcgammaRs modulate the anti-tumor activity of antibodies targeting the PD-1/PD-L1 Axis. Cancer Cell. 2015;28:285–95. doi: 10.1016/j.ccell.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 31.Grenga I, Donahue RN, Lepone LM, Richards J, Schlom J. A fully human IgG1 anti-PD-L1 MAb in an in vitro assay enhances antigen-specific T-cell responses. Clin Transl Immunol. 2016;5:e83. doi: 10.1038/cti.2016.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boyerinas B, Jochems C, Fantini M, Heery CR, Gulley JL, Tsang KY, Schlom J. Antibody-dependent cellular cytotoxicity activity of a novel anti-PD-L1 antibody avelumab (MSB0010718C) on human tumor cells. Cancer Immunol Res. 2015;3:1148–57. doi: 10.1158/2326-6066.CIR-15-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piccione EC, Juarez S, Liu J, Tseng S, Ryan CE, Narayanan C, Wang L, Weiskopf K, Majeti R. A bispecific antibody targeting CD47 and CD20 selectively binds and eliminates dual antigen expressing lymphoma cells. MAbs. 2015;7:946–56. doi: 10.1080/19420862.2015.1062192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu X, Pu Y, Cron K, Deng L, Kline J, Frazier WA, Xu H, Peng H, Fu YX, Xu MM. CD47 blockade triggers T cell-mediated destruction of immunogenic tumors. Nat Med. 2015;21:1209–15. doi: 10.1038/nm.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Minn AJ, Wherry EJ. Combination cancer therapies with immune checkpoint blockade: Convergence on interferon signaling. Cell. 2016;165:272–5. doi: 10.1016/j.cell.2016.03.031. [DOI] [PubMed] [Google Scholar]
- 36.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al.. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, et al.. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–33. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, Bettini ML, Gravano DM, Vogel P, Liu CL, et al.. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72:917–27. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garber K. Bispecific antibodies rise again. Nat Rev Drug Discov. 2014;13:799–801. doi: 10.1038/nrd4478. [DOI] [PubMed] [Google Scholar]
- 41.Junttila TT, Li J, Johnston J, Hristopoulos M, Clark R, Ellerman D, Wang BE, Li Y, Mathieu M, Li G, et al.. Antitumor efficacy of a bispecific antibody that targets HER2 and activates T cells. Cancer Res. 2014;74:5561–71. doi: 10.1158/0008-5472.CAN-13-3622-T. [DOI] [PubMed] [Google Scholar]
- 42.Park S, Jiang Z, Mortenson ED, Deng L, Radkevich-Brown O, Yang X, Sattar H, Wang Y, Brown NK, Greene M, et al.. The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell. 2010;18:160–70. doi: 10.1016/j.ccr.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang X, Zhang X, Mortenson ED, Radkevich-Brown O, Wang Y, Fu YX. Cetuximab-mediated tumor regression depends on innate and adaptive immune responses. Mol Ther. 2013;21:91–100. doi: 10.1038/mt.2012.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang X, Zhang X, Fu ML, Weichselbaum RR, Gajewski TF, Guo Y, Fu YX. Targeting the tumor microenvironment with interferon-beta bridges innate and adaptive immune responses. Cancer Cell. 2014;25:37–48. doi: 10.1016/j.ccr.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu EF, Gai SA, Opel CF, Kwan BH, Surana R, Mihm MC, Kauke MJ, Moynihan KD, Angelini A, Williams RT, et al.. Synergistic innate and adaptive immune response to combination immunotherapy with anti-tumor antigen antibodies and extended serum half-life IL-2. Cancer Cell. 2015;27:489–501. doi: 10.1016/j.ccell.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang Y, Guo Q, Xia M, Li Y, Peng X, Liu T, Tong X, Xu J, Guo H, Qian W, et al.. Generation and characterization of a target-selectively activated antibody against epidermal growth factor receptor with enhanced anti-tumor potency. MAbs. 2015;7:440–50. doi: 10.1080/19420862.2015.1008352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu B, Guo H, Xu J, Qin T, Xu L, Zhang J, Guo Q, Zhang D, Qian W, Li B, et al.. Acid-induced aggregation propensity of nivolumab is dependent on the Fc. MAbs. 2016;8:1107–17. doi: 10.1080/19420862.2016.1197443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu B, Guo H, Zhang J, Xue J, Yang Y, Qin T, Xu J, Guo Q, Zhang D, Qian W, et al.. In-depth characterization of a pro-antibody-drug conjugate by LC-MS. Mol Pharm. 2016;13:2702–10. doi: 10.1021/acs.molpharmaceut.6b00280. [DOI] [PubMed] [Google Scholar]
- 49.Parekh BS, Berger E, Sibley S, Cahya S, Xiao L, LaCerte MA, Vaillancourt P, Wooden S, Gately D. Development and validation of an antibody-dependent cell-mediated cytotoxicity-reporter gene assay. MAbs. 2012;4:310–8. doi: 10.4161/mabs.19873. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


