Abstract
Background/Setting
Randomized controlled trials (RCTs) of HIV biomedical prevention interventions often enroll participants with varying levels of HIV exposure, including people never exposed to HIV. We assessed whether enrolling larger proportion of participants with consistently high exposure to HIV, such as female sex workers (FSW), might reduce trial duration and improve the accuracy of product efficacy estimates in future HIV prevention trials.
Methods
We used an individual-based stochastic model to simulate event-driven RCTs of an HIV prevention intervention providing 80% reduction in susceptibility per act under different proportions of FSW enrolled. A 5% annual drop-out rate was assumed for both FSW and non-FSW in our main scenario, but rates of up to 50% for FSW were also explored.
Results
Enrolling 20% and 50% FSW reduced the median simulated trial duration from 30 months with 0% FSW enrolled to 22 months and 17 months, respectively. Estimated efficacy increased from 71% for RCTs without FSW to 74% and 76% for RCTs with 20% and 50% FSW enrolled, respectively. Increasing the FSW drop-out rate to 50% increased the duration of RCTs by 1–2 months on average and preserved the gain in estimated efficacy.
Conclusion
Despite the potential logistical challenges of recruiting and retaining FSW, trialists should revisit the idea of enrolling FSW in settings where HIV incidence among FSW is higher than among non-FSW. Our analysis suggests that enrolling FSW would increase HIV incidence, reduce trial duration and improve efficacy estimates, even if the annual drop-out rate among FSW participants is high.
Keywords: HIV prevention, clinical trial, female sex workers, mathematical modeling
Introduction
New biomedical modalities for HIV prevention are currently undergoing testing in randomized controlled trials (RCTs) among individuals believed to be at-risk for HIV. The design of these RCTs typically assumes a common effectiveness at all levels of HIV acquisition risk, irrespective of exposure level. However, if participants with low or no HIV exposure are enrolled –a group in which an effective intervention cannot demonstrate an effect – then attenuation of observed treatment efficacy and thereby reduced efficiency of RCTs will occur. Here we explore the extent to which enrolling large fractions of participants from populations with consistently high exposure to HIV, such as female sex workers (FSW), may improve the efficiency of an RCT and more accurately estimate product efficacy in future HIV prevention trials.
Globally, FSW are disproportionately infected with HIV.[1] Within sub-Saharan Africa, the HIV prevalence among FSW is nearly 40% [2] with regional estimates of approximately 70%.[3, 4] Annual HIV incidence among FSW in sub-Saharan Africa ranges from 0.90 per 100 person-years (95% CI: 0.24, 1.58) in Burkina Faso to 9.8 (95% CI: 7.10, 15.90) in Zimbabwe.[5–14] Despite FSW being at high risk for HIV acquisition, few recent HIV prevention trials have purposely enrolled HIV uninfected FSW.[15] Most large, multisite HIV prevention trials targeting women do not report the proportion of study participants practicing sex work or transactional sex, yet of those trials that do, the majority report less than 10% engaging in such practices.[15–17] Given their considerable risk of HIV acquisition, FSWs would clearly benefit from effective prevention interventions and thus should be included in prevention trials.
Our previous modeling analysis [18] suggested that the majority of women enrolled in HIV prevention trials in high-prevalence settings remain unexposed to HIV. If exposure to HIV is concentrated in a relatively small but unmeasured fraction of trial participants, this unobservable heterogeneity in HIV acquisition risk will introduce frailty selection bias – where only participants who have infected partners acquire HIV. Women with a low rate of partnership exchange are either repeatedly or never exposed to HIV during follow up, depending on the HIV status of their partners. Therefore, the HIV incidence among repeatedly exposed women will be substantially higher than the overall incidence in the cohort. If a highly effective prevention product is tested, the risk of infection will be significantly reduced among exposed women in the active arm. Because highly exposed individuals will become infected and removed from follow-up more quickly in the placebo than in the active arm, there will be a growing imbalance in HIV exposure risk between trial arms over time, even if the arms were perfectly balanced at the time of randomization.[19] As a result, the product efficacy estimated in RCTs with concentrated HIV exposure is lower compared to trials with increased prevalence of HIV exposure.[19, 20] Enrolling high-risk women who in behavioral questionnaires report more frequent change of partners may increase the proportion of participants exposed to HIV in settings with high male HIV prevalence. However, it is unlikely to reduce substantially the imbalance in HIV exposure because the majority of high-risk women still have large number of sex acts with few partners. We argue that enrolling FSW, who have few sex acts with a large number of partners, will have more profound effect on the trial results.
Here we explore the extent to which enrolling different proportions of FSW may reduce the expected duration of trials as well as limit attenuation of efficacy estimates, and thereby increase the likelihood of correctly identifying effective biomedical products. We compared scenarios in which FSW have a high volume of clients and consistent condom use (e.g. professional FSWs) with scenarios in which FSWs practice sex work less frequently with fewer clients (e.g. part time FSWs). Although we hypothesize that enrolling FSWs may improve trial efficiency, retaining FSWs in trials over long follow-up periods may be challenging. Thus, we additionally investigated the influence on trial duration of higher loss-to-follow-up rates in FSWs vs. non-FSW.
Methods
Overview
We modified a previously published stochastic individual-based model [18, 21] to simulate randomized placebo-controlled trials of HIV prevention interventions in women, assuming enrollment of different FSW proportions. The model was designed to reproduce the sexual behavior of a cohort of 3600 sexually active HIV-uninfected women in high HIV prevalence settings, accrued using the enrollment criteria of the recently concluded MTN 020 ASPIRE trial [22] over a one-year period and randomized in a 1:1 ratio to active or placebo arms. We simulated two types of RCTs (Table 1). The primary design of interest was an event-driven trial, i.e., a trial that concludes when a pre-specified number of infections have occurred. The secondary design of interest (explored in the Supplemental Digital Content) was an RCT where all participants were followed for a fixed period of 3 years.
Table 1. Description of all scenarios explored in the analysis.
Values in bold are used in the main set of simulations. Remaining values are explored in secondary scenarios for sensitivity analyses.
| Parameter varied | Values explored |
| RCT design |
Event-driven trial targeting 120 infections, Trial with uniform 3-year follow up |
| True efficacy per act (% reduction of susceptibility per HIV-exposed act) | 50%, 80% |
| Percentage of participants who are FSW | 0%, 20%, 50%, 100% |
| HIV incidence among FSW due to sex work |
High (4–8%). Overall incidence among FSW 7–12%. Low (1–2%). Overall incidence among FSW 3–5%. |
| Loss-to-follow-up rates (annually) among non-FSW | 5% |
| Loss-to-follow-up rates (annually) among FSW | 5%, 10%, 20%, 50% |
The women in the cohort were divided into three categories: FSW, low-risk non-FSW, and high-risk non-FSW, with low-risk and high-risk groups defined by propensity for concurrent partnerships. Every woman (FSWs and non-FSWs) in the main scenario could be involved in two types of steady partnerships: i) short-term with expected duration 6–12 months in which condoms were used more frequently; ii) long-term with expected duration of 10 years in which condoms were used less frequently. The partners’ and clients’ characteristics, their baseline HIV and treatment status as well as their risk of HIV acquisition outside of the partnership were simulated according to data-derived parameters from published studies on sexual behavior patterns and HIV transmission in South Africa. [23–25] The frequency of sexual acts was assigned at the initiation of each partnership and remained constant for its duration. Similar coital frequency was assumed for long- and short-term couples with sexual acts occurring randomly in time. An average of 20% of all partnerships were assumed to practice anal sex and with an average of 40% of all sex acts to be anal. [23]
A complete description of the model, model parameters and the values used in the analysis is included in the Supplemental Digital Content.
HIV transmission and prevention
The HIV acquisition risk per sex act was determined by the stage of infection (acute, asymptomatic or late) of the infected partner or client, his treatment status, the type of act (vaginal or anal), and the use or non-use of a condom. The model was calibrated to reproduce annual HIV incidence from sex work in the range 4–8% among FSW.
The efficacy of imperfect biological interventions can be conceptualized as either partially protective in all people or fully protective in only a proportion of people. We assumed partial protection to everybody using the product at every HIV exposure [26–28]. For simplicity, we refer to this protection as the “true efficacy per act” in this paper. In our main scenario, we simulated event-driven RCTs of a biomedical intervention with a time-invariant 80% true efficacy per act (reducing susceptibility to HIV infection per sexual contact with an HIV-infected man). Alternatively, we assessed the potential importance of FSW enrollment when the product is only moderately effective (50% true efficacy per act). The “estimated efficacy” in the RCTs was calculated as one minus the incidence rate ratio (IRR) of acquiring HIV, defined as the ratio of the HIV incidence rate in the active vs control arm.
Trial Simulations and Outcomes of Interest
We explored enrollment of different proportions of FSW (0%, 20%, 50% and 100%) assuming 9.5% median HIV incidence among FSW and 2.8% median annual HIV incidence among non-FSW in the cohort (Table 1). The annual retention rate target of 95% (5% drop-out rate) was used in the main scenario among both FSW and non-FSW. To assess the importance of FSW retention we considered alternative scenarios with drop-out rates up to 50% among FSW.
The main outcomes of interest were trial duration and estimated efficacy in the simulated RCTs. We also compared the annual HIV incidence and total follow-up time by arm across scenarios. Outcomes were compared across scenarios with different proportions of FSW enrolled. For each scenario, we present median value, interquartile range and 90% uncertainty interval (UI) based on 1000 RCTs simulated per scenario.
Sensitivity analyses
We conducted additional analyses to help understand the impact of FSW enrollment in RCTs. We investigated alternative scenarios in which recruited FSW had less frequent contacts with clients resulting in substantially lower HIV incidence (1–2%) due to sex work. We compared cohorts with the same overall HIV incidence rate in the control arm but different proportion of FSW enrolled. We also simulated RCTs where participants were enrolled simultaneously and followed for a fixed duration of 3 years.
Results
Impact of FSW enrollment on HIV Incidence
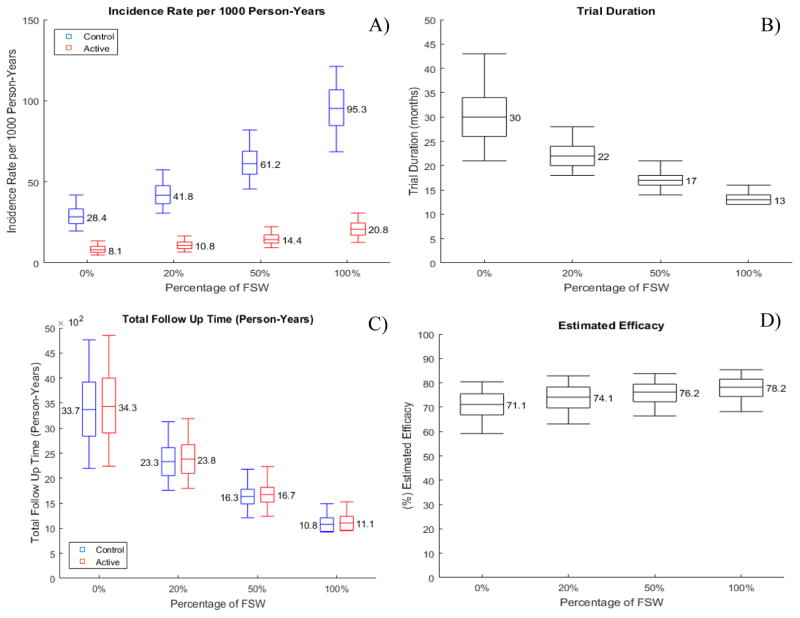
In the simulated trials, enrolling professional FSW participants resulted in increasing HIV incidence rates (Figure 1A). For a cohort without FSW, the median annual HIV incidence was 2.8% in the control arm. Simulated trials with 20% FSW participants resulted in a median annual HIV incidence of 4.2% in the control arm. The median annual HIV incidence within the control arm further increased to 6.1% with 50% FSW participants enrolled and to 9.5% with a cohort of all FSW participants. Within the active intervention arm, the median annual HIV incidence was 0.8% among a cohort without FSW. Enrolling 20% and 50% FSW participants increased the median annual HIV incidence to 1.1% and 1.4% respectively in the active arm. Simulated trials with all FSW participants resulted in a median annual HIV incidence in the active arm of 2.1%.
Figure 1. Projected impact of enrolling professional FSW in prevention RCTs.
A) Projected HIV incidence rate by arm; B) Trial duration; C) Total follow-up time by arm; D) Estimated efficacy. Fixed true efficacy of 80% in reducing HIV susceptibility per exposed act and 5% annual dropout rate are assumed over the course of the trial. Box plots and whiskers represent quartile ranges and 90% UI, reflecting the estimated variation over 1000 trials simulated.
Impact of FSW enrollment on trial duration for different HIV incidence rates
Our simulations suggested that enrolling professional FSW participants substantially reduced the expected trial duration (Figure 1B) due to the overall increase in HIV incidence. When compared to trials without FSW, enrolling 20% FSW reduced the median trial duration by 27% from 30 months to 22 months. The total follow-up time needed to reach the targeted number of infections was reduced by 31% from 6800 person-years to 4710 person-years (Fig. 1C). Under the simulated scenarios with targeted FSW enrollment, 76% of the trials enrolling 20% FSW were completed within 2 years after the start of enrollment compared to only 18% when no FSW were enrolled. The trial duration was further shortened as the proportion of enrolled FSW increased. With 50% FSW, the median trial duration was 17 months, with 100% of the simulated trials coming to completion within 2 years. The total expected follow-up time in this scenario was reduced by more than 50% compared to simulations with no FSW. Among the trials where only FSW participants were enrolled, the median trial duration was 13 months.
The extent to which trial duration was shortened was highly dependent on the difference in HIV incidence among FSW and non-FSW populations because it determined the increases in the overall HIV incidence when more FSW were enrolled. Simulated RCTs in which FSW incidence (4%) was only marginally higher than the non-FSW incidence (2.8%) demonstrated that enrolling 20% and 50% FSW shortened trial duration by only 1 and 3 months in comparison with trials without FSW (Fig. S1).
Impact of FSW enrollment on estimated efficacy in RCTs
Enrolling FSW may provide additional advantages when the biomedical intervention is not perfect (true efficacy per act <1) by reducing the extent to which efficacy estimated in RCTs underestimates the true efficacy resulting from frailty effects. The estimated efficacy of the HIV prevention intervention was projected to increase as the proportion of enrolled FSW participants increased (Figure 1D). In trials without FSW participants the median estimated efficacy was 71% (90% UI: 62.4%–78.8%), nearly 10 percentage points below the assumed true efficacy per act of 80%. When enrolling 20% or 50% FSW participants, the estimated efficacy increased to 74% (90% UI: 65.5%–80.1%) or 76% (90% UI: 68.6%–82.4%), respectively. In trials where only FSW participants were enrolled, the median estimated efficacy was 78% (90% UI: 70.4%–84.3%) with the residual difference from 80% due to HIV exposure in steady partnerships. Alternative scenarios assuming that FSW had no partners other than clients showed average estimated efficacy equal to the true efficacy per act (Fig. S2A)
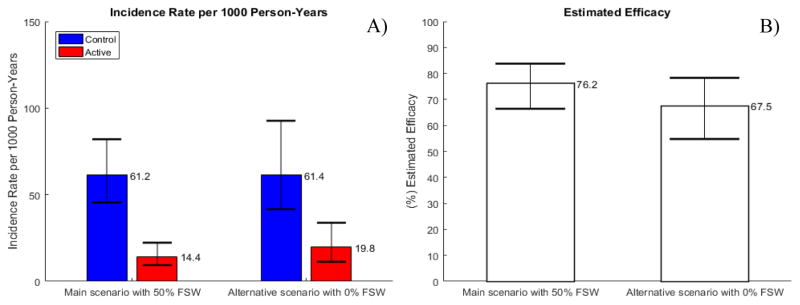
In contrast to trial duration, differences in estimated efficacy cannot be attributed to higher HIV incidence among FSW. We simulated a cohort with 0% FSW in which the control arm HIV incidence was 61 infections per 1000 persons years, identical to the control arm incidence in the main scenario with 50% FSW participants (Fig. 2A). Although both trials were projected to have a similar duration of 17 months, the estimated efficacy was still 9 percentage points lower in the trials with no FSW (Fig. 2B). Additional sensitivity analyses confirmed that increased prevalence of participants with consistently high HIV exposure is the likely reason for the improved efficacy estimates in trials enrolling FSWs rather than differences in HIV incidence rates between FSW and non-FSW and shorter trial duration. Scenarios assuming comparably low HIV incidence of 2.8% among FSW, non-FSW, and FSW having no partners other than clients also showed differences in efficacy estimates up to 9 percentage points due to FSW enrollment (Fig. S2). Differences in estimated efficacy remained unaffected if all participants were simultaneously enrolled and followed for a fixed duration of 3 years (Fig. S3).
Figure 2. Simulations of trials without FSW and 50% FSW with similar overall HIV incidence in the control arm.
A) HIV incidence rate by arm; B) Estimated efficacy in RCT. Fixed true efficacy of 80% in reducing HIV susceptibility per exposed act and 5% annual dropout rate are assumed over the course of the trial. Bars and range plots represent median estimates and 90% UI, reflecting the estimated variation over 1000 trials simulated.
How important is it to retain FSW in the trial?
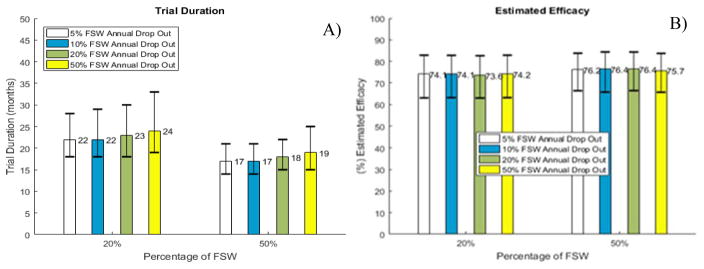
Projected improvements in trial duration and estimated efficacy in RCTs were mostly retained in sensitivity analyses where we modeled higher dropout rates among FSW participants, assuming dropout was independent of arm. Scenarios with 20% and 50% FSW participants showed that HIV incidence in the control arm was reduced slightly when dropout rates among FSW increased to as much as 50% (Fig. S4). RCTs were expected to continue only 2 months longer when 50% of FSW were lost to follow-up annually compared to 5% in the main scenario (Fig. 3A). The likelihood that the RCT with 20% FSW participants enrolled would continue longer than 2 years increased from 24% to 30% when 20% instead of 5% FSW were lost to follow-up annually. The estimated efficacy was essentially unaffected by the lower retention rate provided that women who dropped out were well balanced between arms (Fig. 3B).
Figure 3. Simulations of trials with different dropout rates among FSW.
A) Trial duration and B) Estimated efficacy in RCT. Fixed true efficacy of 80% in reducing HIV susceptibility per exposed act and 5% annual dropout rate among non-FSW are assumed over the course of the trial. Bars and range plots represent median estimates and 90% UI, reflecting the estimated variation over 1000 trials simulated.
What if the true efficacy of the biomedical study product is lower?
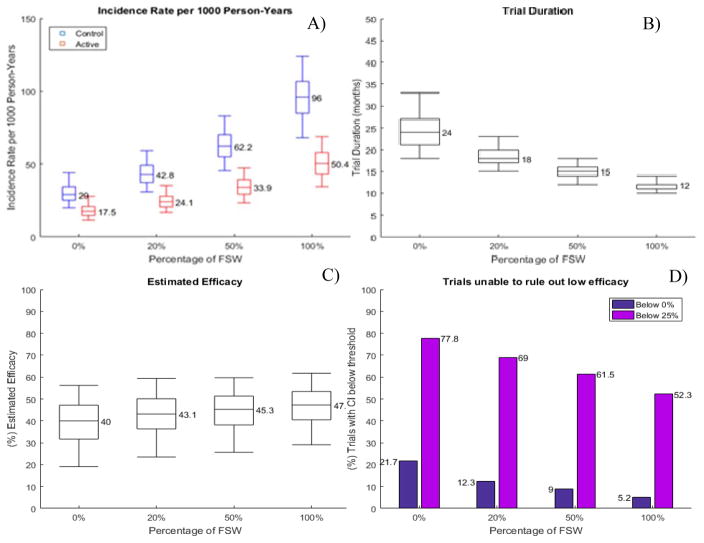
We also assessed the benefits of enrolling FSW in scenarios where true efficacy per act was 50% compared to our main scenario where the true efficacy was fixed at 80% (Fig. 4). As expected, lower true efficacy per act led to higher HIV incidence in the active arms of the simulated trials and as a result RCTs were completed faster even without FSW participants. Enrolling 20% and 50% high-risk FSW further shortened the trial by 25% and 38%, respectively. The estimated efficacy in RCTs without FSW was 40%, which is 10 percentage points lower than true efficacy, a relative loss of 20%. Almost 22% of the RCTs without FSW were expected to report non-significant product efficacy and almost 80% of the RCTs were unable to rule out efficacy below 25%, a predefined goal of prevention trials such as VOICE, Partners PrEP and ASPIRE.[29–31] If 20% and 50% of the participants were FSW, efficacy estimates improved to 43% and 45% and the proportion of inconclusive trials was reduced to 12% and 9%, respectively (Fig. 4D).
Figure 4. Projected impact of FSW enrollment in RCTs assuming 50% true efficacy per exposed act.
A) projected HIV incidence rate by arm; B) projected trial duration; C) estimated efficacy; D) proportion of simulated trials in which the 95% CI of the efficacy estimate includes 0 or the predefined low efficacy limit of 25%. 5% annual dropout rate is assumed over the course of the trial. Box plots and whiskers represent quartile ranges and 90% UI, reflecting the estimated variation over 1000 trials simulated.
Discussion
Successful and timely completion of an event-driven RCT depends on sufficiently high HIV incidence within the enrolled cohort. All HIV prevention RCTs include enrollment criteria designed to ensure that study subjects demonstrate sufficient HIV acquisition risk to justify enrollment. To this end, our model shows that including a substantial number of high-risk FSW in an HIV prevention trial will lead to a significant reduction in trial duration.
More importantly, our model predicted that enrolling FSW with consistently high exposure to HIV would also provide a more accurate estimate of the efficacy of an intervention. Ideally we would be able to quantify the reduction in the risk of HIV acquisition per HIV-exposed sex act, a measure that would be independent of the sexual behavior of each individual. Efficacy estimates from RCTs based on the cumulative incidence ratio (CIR) or incidence rate ratio (IRR) underestimate the true efficacy per act of an imperfect biomedical prevention for at least two reasons. First, a high magnitude of exposure, defined as a high number of HIV exposures per exposed participant, may reduce the observed CIR-based efficacy because the risk of infection accumulates with each additional challenge in both the biomedical product and the placebo arms. [32] The IRR-based efficacy (as used in our analysis) theoretically provides a more accurate estimate of true efficacy because it accounts for the differences in follow up time between arms. However, downward biases also incur with IRR-based efficacy, particularly if testing intervals are long and the frequency of sex acts is high. Second, the concentration of HIV exposure in only a portion of trial participants may attenuate CIR- and IRR-based efficacy estimates due to inclusion of persons with no exposure to HIV, to whom the biomedical product provides no actual protection. Our analysis confirmed that efficacy estimated in RCTs with HIV exposure concentrated in only a portion of participants is lower compared to trials in cohorts with more evenly distributed HIV exposure. [19, 20]
Improved estimates of product efficacy in RCTs with more FSW enrolled cannot be attributed to increased overall HIV incidence in the cohort, but rather to larger proportions of participants with consistently high HIV exposure. Improved efficacy estimates provide an additional benefit by reducing the likelihood of the RCT to report inconclusive results, especially when the product is moderately effective in reducing the risk of HIV infection per exposed sex act.
Given FSW in some settings are a highly mobile population, consideration must be given to the potential for low retention of FSW in RCTs. In trials such as FEM-PrEP, which included approximately 10% of enrolled women reporting sex work or transactional sex at baseline, retention was over 85%. [16] Similarly, an HIV-negative cohort of South African women in which 79% reported sex work and transactional sex at baseline had 87% retention among all participants. [12] Our analysis suggested that most of the reductions in trial duration and efficacy biases afforded by FSW enrollment will be preserved even if drop-out rates among FSW are high. Within our model, which assumed FSW were missing at random, up to 50% annual loss to follow up among FSW participants had little effect on the trial outcomes because its negative impact on duration and accuracy was compensated by the positive impact of enrolling participants with higher HIV incidence and equally exposed to HIV. Despite that the potential for poor retention in RCTs is always a concern and therefore enrollment of FSW into trials must be accompanied by efforts to maximize retention.
The support our study provides for FSW-focused RCT enrollment has several caveats. First, not all FSWs have the same HIV risk. In Zimbabwe, estimated HIV incidence among FSW is 10–12 times higher than in the general population. [33, 34] However, in India, Thailand, and Benin, targeted scale-up of condom interventions has reduced the risk of HIV in FSW to levels comparable with that of the general population. [35, 36] In some high HIV-prevalence settings the HIV incidence among women enrolled in previous trials was already so high (nearly 10%) that enrolling FSW would not necessary benefit a trial. [37] Our analysis suggested that the extent to which enrolling FSWs may reduce trial duration by increasing HIV incidence depends on the difference in HIV incidence among FSWs and non-FSWs. Second, enrolling FSW could result in poor product performance due to factors associated with extreme exposure to HIV, leading to rejection of a prevention product that would have reasonable benefit to a lower risk population. FSW frequently engaging in unprotected sex with clients are more likely to be exposed to HIV variants resistant to the prevention product compared to non-FSW. Other STDs that cause inflammation, common in FSW, may also reduce product efficacy. Finally, highly sexually active FSW are more likely to be exposed to HIV during a window of time when product concentration falls below a protective threshold, such as when long-lasting prevention products are tested. That may lead to apparent product failure, highlighting the need for pre-specified sub-group analysis.
The ability to recruit a substantial number of FSW in trials will depend on the size of the FSW population in the setting of interest, their relationship with the larger community and the existence of programs which offer other HIV prevention services to FSW. Therefore, site-specific data with reliable population size estimates of FSW and information about local epidemiological, demographic, social and behavioral factors would provide important insights on effective methods for recruiting FSW at risk for HIV.
Our study has several limitations. We did not account for the disclosure of HIV status by male partners and its influence on sexual behavior, condom use and adherence to HIV prevention products. Results from RCTs of oral PrEP in sero-discordant couples and in individually enrolled women suggest that knowledge of the partners’ HIV status could be a strong incentive to take PrEP consistently. The likelihood of suboptimal PrEP adherence among FSW is uncertain. One trial, FEM-PrEP, reported no significant relationship between transactional sex and PrEP adherence. [15] Including FSW in future trials will allow for addressing the question of differential adherence and acceptability between FSW and non-FSW and performing subgroup analyses within the same epidemic settings. At present, our analysis may be most relevant to RCTs with directly observed therapy, such as long-lasting injections and monoclonal antibody infusions, where adherence within trial cohorts is not an issue.
Important in this strategy, FSW and other local populations would greatly benefit from novel HIV prevention products that prove successful in an RCT; in many settings, HIV infected FSWs offer disproportionate risk for the spread of HIV so prevention of infection in this population is particularly important. Regardless of the potential challenges of recruiting and retaining FSW, enrolling FSW in HIV prevention trials has considerable advantages for trial design and validity, along with benefits for the study subjects. Our analysis quantifies these potential trial advantages, including faster completion, better estimates of the true product efficacy, and a reduced likelihood for inconclusive results.
Supplementary Material
Acknowledgments
The authors are grateful to Thea Swanson, Executive Assistant at the Vaccine and Infectious Disease Division of Fred Hutchinson Cancer Research Center for proofreading and editing the final version of the manuscript.
Funding: DW, KEL, MCB, DD, MSC and DTD acknowledge the NIH support to the HIV Prevention Trials Network (UM1 AI068617 and 5UM1AI068619-11). KEL was also supported by 5U01AI069423-04.
Footnotes
KEL, MCB, MSC and DTD designed the study. DW and KEL collected data and DW carried out the modeling analysis. DW, KEL and DTD drafted the article. KAP, DD, MCB and MSC contributed data to the study, interpreted the results and revised the article. All authors reviewed the final draft of the article and approved for submission.
Presentations: No parts of this work have been presented at scientific meetings.
References
- 1.UNAIDS. Global HIV/AIDS Report. Geneva, Switzerland: United Nations; 2013. [Google Scholar]
- 2.Baral S, Beyrer C, Muessig K, Poteat T, Wirtz AL, Decker MR, et al. Burden of HIV among female sex workers in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:538–549. doi: 10.1016/S1473-3099(12)70066-X. [DOI] [PubMed] [Google Scholar]
- 3.UCSF, Anova Health Institute, WRHI. Final Report: The Integrated Biological and Behavioural Survey among Female Sex Workers, South Africa 2013–2014. San Francisco: UCSF; 2015. [Google Scholar]
- 4.Lancaster KE, Powers KA, Lungu T, Mmodzi P, Hosseinipour MC, Chadwick K, et al. The HIV Care Continuum among Female Sex Workers: A Key Population in Lilongwe, Malawi. PLoS One. 2016;11:e0147662. doi: 10.1371/journal.pone.0147662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Konate I, Traore L, Ouedraogo A, Sanon A, Diallo R, Ouedraogo JL, et al. Linking HIV prevention and care for community interventions among high-risk women in Burkina Faso--the ARNS 1222 “Yerelon” cohort. J Acquir Immune Defic Syndr. 2011;57(Suppl 1):S50–54. doi: 10.1097/QAI.0b013e3182207a3f. [DOI] [PubMed] [Google Scholar]
- 6.Watson-Jones D, Baisley K, Weiss HA, Tanton C, Changalucha J, Everett D, et al. Risk factors for HIV incidence in women participating in an HSV suppressive treatment trial in Tanzania. Aids. 2009;23:415–422. doi: 10.1097/QAD.0b013e32831ef523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graham SM, Raboud J, McClelland RS, Jaoko W, Ndinya-Achola J, Mandaliya K, et al. Loss to follow-up as a competing risk in an observational study of HIV-1 incidence. PLoS One. 2013;8:e59480. doi: 10.1371/journal.pone.0059480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Price MA, Rida W, Mwangome M, Mutua G, Middelkoop K, Roux S, et al. Identifying at-risk populations in Kenya and South Africa: HIV incidence in cohorts of men who report sex with men, sex workers, and youth. J Acquir Immune Defic Syndr. 2012;59:185–193. doi: 10.1097/QAI.0b013e31823d8693. [DOI] [PubMed] [Google Scholar]
- 9.Luchters S, Richter ML, Bosire W, Nelson G, Kingola N, Zhang XD, et al. The contribution of emotional partners to sexual risk taking and violence among female sex workers in Mombasa, Kenya: a cohort study. PLoS One. 2013;8:e68855. doi: 10.1371/journal.pone.0068855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Priddy FH, Wakasiaka S, Hoang TD, Smith DJ, Farah B, del Rio C, et al. Anal sex, vaginal practices, and HIV incidence in female sex workers in urban Kenya: implications for the development of intravaginal HIV prevention methods. AIDS Res Hum Retroviruses. 2011;27:1067–1072. doi: 10.1089/aid.2010.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vandepitte J, Weiss HA, Bukenya J, Nakubulwa S, Mayanja Y, Matovu G, et al. Alcohol use, mycoplasma genitalium, and other STIs associated With HIV incidence among women at high risk in Kampala, Uganda. J Acquir Immune Defic Syndr. 2013;62:119–126. doi: 10.1097/QAI.0b013e3182777167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Loggerenberg F, Mlisana K, Williamson C, Auld SC, Morris L, Gray CM, et al. Establishing a cohort at high risk of HIV infection in South Africa: challenges and experiences of the CAPRISA 002 acute infection study. PLoS One. 2008;3:e1954. doi: 10.1371/journal.pone.0001954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braunstein SL, Ingabire CM, Kestelyn E, Uwizera AU, Mwamarangwe L, Ntirushwa J, et al. High human immunodeficiency virus incidence in a cohort of Rwandan female sex workers. Sex Transm Dis. 2011;38:385–394. doi: 10.1097/olq.0b013e31820b8eba. [DOI] [PubMed] [Google Scholar]
- 14.Mishra S. Using mathematical models to characterize HIV epidemics for the design of HIV prevention strategies. Imperial College; London: 2014. [Google Scholar]
- 15.Bekker LG, Johnson L, Cowan F, Overs C, Besada D, Hillier S, et al. Combination HIV prevention for female sex workers: what is the evidence? Lancet. 2015;385:72–87. doi: 10.1016/S0140-6736(14)60974-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367:411–422. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El-Sadr W HPTN State of the Network. HIV Prevention Trials Network (HPTN)/International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) Network Annual Meeting; Washington, DC. 2015. [Google Scholar]
- 18.Dimitrov D, Donnell D, Brown ER. High Incidence Is Not High Exposure: What Proportion of Prevention Trial Participants Are Exposed to HIV? PLoS ONE. 2015;10:e0115528. doi: 10.1371/journal.pone.0115528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desai KN, Boily MC, Masse BR, Alary M, Anderson RM. Simulation studies of phase III clinical trials to test the efficacy of a candidate HIV-1 vaccine. Epidemiology and Infection. 1999;123:65–88. doi: 10.1017/s0950268899002642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Auvert B, Sitta R, Zarca K, Mahiane SG, Pretorius C, Lissouba P. The effect of heterogeneity on HIV prevention trials. Clinical Trials. 2011;8:144–154. doi: 10.1177/1740774511398923. [DOI] [PubMed] [Google Scholar]
- 21.Dimitrov DT, MÂsse BR, Donnell D. PrEP adherence patterns strongly impact individual HIV risk and observed efficacy in randomized clinical trials. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2016 doi: 10.1097/QAI.0000000000000993. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Microbicide Trials Network. MTN-020 A Multi-Center, Randomized, Double-Blind, Placebo-Controlled Phase 3 Safety and Effectiveness Trial of a Vaginal Matrix Ring Containing Dapivirine for the Prevention of HIV-1 Infection in Women. 2011 http://www.mtnstopshiv.org/sites/default/files/attachments/MTN-020%20Version1%200_28September2011_CLEAN.pdf.
- 23.Kalichman SC, Simbayi LC, Cain D, Jooste S. Heterosexual anal intercourse among community and clinical settings in Cape Town, South Africa. Sexually Transmitted Infections. 2009;85:411–415. doi: 10.1136/sti.2008.035287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson L, Dorrington R, Bradshaw D, Pillay-Van Wyk V, Rehle T. Sexual behaviour patterns in South Africa and their association with the spread of HIV: insights from a mathematical model. Demographic Research. 2009;21:289–340. [Google Scholar]
- 25.Shisana OTR, Simbayi LC, Zuma K, Jooste S, Mbelle N, Van Zyl J, Pezi S, Parker W, Zungu NP, Pillay-Van Wyk V. South African national HIV prevalence, incidence, behaviour and communication survey, 2008: a turning tide among teenagers? - HSRC Press; 2009. [Google Scholar]
- 26.Hallett TB, Baeten JM, Heffron R, Barnabas R, de Bruyn G, Cremin I, et al. Optimal Uses of Antiretrovirals for Prevention in HIV-1 Serodiscordant Heterosexual Couples in South Africa: A Modelling Study. Plos Medicine. 2011;8:e1001123. doi: 10.1371/journal.pmed.1001123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beyrer C, Baral SD, van Griensven F, Goodreau SM, Chariyalertsak S, Wirtz AL, et al. Global epidemiology of HIV infection in men who have sex with men. Lancet. 2012;380:367–377. doi: 10.1016/S0140-6736(12)60821-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stover J, Hallett TB, Wu Z, Warren M, Gopalappa C, Pretorius C, et al. How Can We Get Close to Zero? The Potential Contribution of Biomedical Prevention and the Investment Framework towards an Effective Response to HIV. Plos One. 2014;9:e111956. doi: 10.1371/journal.pone.0111956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, et al. Tenofovir-Based Preexposure Prophylaxis for HIV Infection among African Women. New England Journal of Medicine. 2015;372:509–518. doi: 10.1056/NEJMoa1402269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral Prophylaxis for HIV Prevention in Heterosexual Men and Women. New England Journal of Medicine. 2012;367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baeten JM, Palanee-Phillips T, Brown ER, Schwartz K, Soto-Torres LE, Govender V, et al. Use of a Vaginal Ring Containing Dapivirine for HIV-1 Prevention in Women. N Engl J Med. 2016 doi: 10.1056/NEJMoa1506110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson DP. Interpreting sexually transmissible infection prevention trials by adjusting for the magnitude of exposure. Clinical Trials. 2010;7:36–43. doi: 10.1177/1740774509355177. [DOI] [PubMed] [Google Scholar]
- 33.Hargreaves JR, Mtetwa S, Davey C, Dirawo J, Chidiya S, Benedikt C, et al. Implementation and Operational Research: Cohort Analysis of Program Data to Estimate HIV Incidence and Uptake of HIV-Related Services Among Female Sex Workers in Zimbabwe, 2009–2014. J Acquir Immune Defic Syndr. 2016;72:e1–8. doi: 10.1097/QAI.0000000000000920. [DOI] [PubMed] [Google Scholar]
- 34.Ministry of Health and Care. Zimbabwe National HIV and AIDS Estimates Report 2013. Harare, Zimbabwe: Ministry of Health and Child Care; 2013. [Google Scholar]
- 35.Boily MC, Pickles M, Lowndes CM, Ramesh BM, Washington R, Moses S, et al. Positive impact of a large-scale HIV prevention programme among female sex workers and clients in South India. Aids. 2013;27:1449–1460. doi: 10.1097/QAD.0b013e32835fba81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams JR, Alary M, Lowndes CM, Béhanzin L, Labbé A-C, Anagonou S, et al. Positive Impact of Increases in Condom Use among Female Sex Workers and Clients in a Medium HIV Prevalence Epidemic: Modelling Results from Project SIDA1/2/3 in Cotonou, Benin. PLoS ONE. 2014;9:e102643. doi: 10.1371/journal.pone.0102643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balkus JE, Nair G, Montgomery ET, Mishra A, Palanee-Phillips T, Ramjee G, et al. Age-Disparate Partnerships and Risk of HIV-1 Acquisition Among South African Women Participating in the VOICE Trial. Jaids-journal of Acquired Immune Deficiency Syndromes. 2015;70:212–217. doi: 10.1097/QAI.0000000000000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.