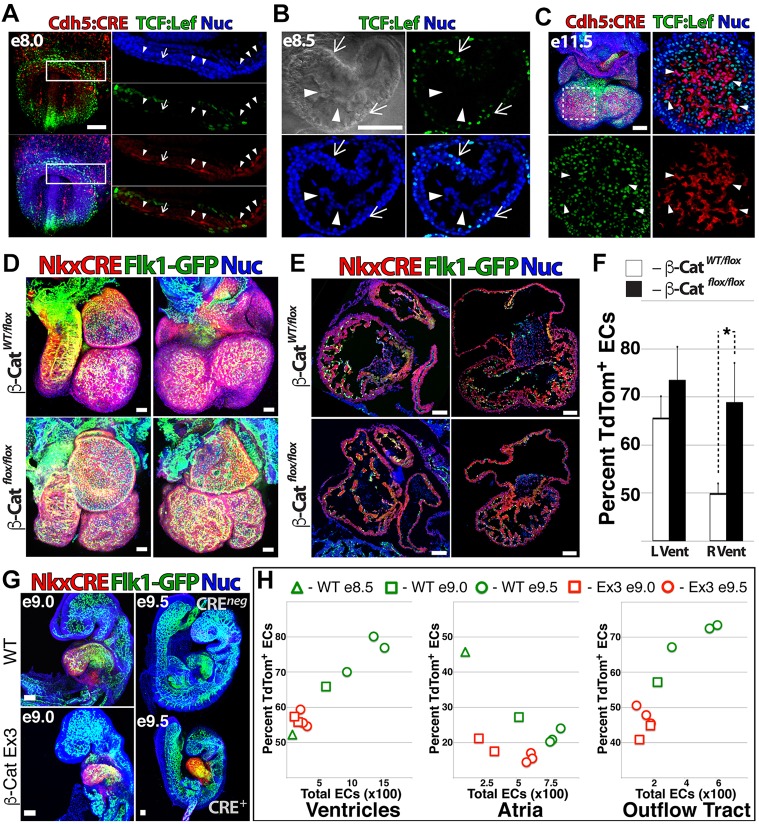
Fig. 5.
Cardiac-specific gain/loss of Wnt function modulates vascular fate in vivo. (A-C) Whole-mount confocal images of TCF/Lef:H2B-GFP;Cdh5Cre;RosatdTom mouse embryos at E8.0 (A) and E8.5 (B) and isolated heart at E11.5 (C). Arrowheads (A-C) indicate ECs; arrows indicate (A) an EC that is faintly positive for TCF/Lef:H2B-GFP or (B,C) TCF/Lef:H2B-GFP+ cells. (D,E) The β-cateninLOF strain was crossed with Flk1-GFP;Nkx2.5Cre;RosatdTom mice to generate embryos with heterozygous and homozygous ablation of β-catenin. z-stack projections are compared (D) and sagittal and coronal sections are shown (E). (F) The percentage of NkxECs was quantified for the left and right ventricles of β-cateninLOF versus heterozygous littermates. Error bars represent s.d. between five biological replicates. *P<0.05. (G,H) β-cateninGOF males were crossed with Flk1-GFP;Nkx2.5Cre;RosatdTom females to generate embryos with constitutively active Wnt signaling in the heart. β-cateninGOF embryos versus control littermates are shown at E9.0 and E9.5 (G) and total EC number and percentage NkxECs were measured for ventricles, atria and outflow tract of β-cateninGOF versus control littermate hearts between E8.5 and E9.5 (H). Scale bars: A-E,G, 100 µm.