ABSTRACT
Cycloserine (Cs) is recommended by the World Health Organization as a second-line drug to treat multidrug-resistant tuberculosis (MDR-TB); however, its efficacy has never been sufficiently evaluated. To gain some insights into the value of cycloserine for MDR-TB treatment, in vitro bacteriostatic effect was determined and patient validations were performed prospectively. The in vitro activity of Cs against 104 wild-type Mycobacterium tuberculosis strains was determined, and serum Cs concentrations were measured for 73 MDR TB patients 2 h after administration. The treatment outcomes for 27 MDR-TB patients who had baseline isolates and were treated with Cs-containing regimens were followed up. The MICs for 90% of the recruited 104 wild-type strains were below 32 μg/ml. Eighteen out of 52 patients had peak serum concentrations (Cmax) below 20 μg/ml at the dosage of 500 mg daily, while 13 out of 21 patients had peak serum concentrations higher than 35 μg/ml at the dosage of 750 mg daily. The percentage of favorable treatment outcomes among patients with a Cmax/MIC ratio of ≥1 was statistically significantly higher than that among the group with a Cmax/MIC ratio of <1 (P = 0.022). The epidemiological cutoff value for Cs susceptibility testing was 32 μg/ml. A high percentage of patients receiving the recommended dosage of 10 mg/kg for Cs administration could not acquire desirable blood concentrations; therefore, adjusting the dosage according to drug concentration monitoring is necessary. The Cmax/MIC ratio might be a good indicator for predicting the treatment outcome for patients with MDR-TB or extensively drug-resistant TB (XDR-TB) who are being administered Cs-containing regimens.
KEYWORDS: cycloserine, efficacy, extensive drug resistance, multiple drug resistance, therapeutic drug monitoring, tuberculosis
INTRODUCTION
Multidrug-resistant tuberculosis (MDR-TB), especially extensively drug-resistant tuberculosis (XDR-TB), is a big challenge for public health (1, 2). Severely drug-resistant TB is often associated with a poor clinical outcome (3, 4). The development of new highly active anti-TB agents and optimal use of currently available drugs are compelling needs.
As an oral bacteriostatic antituberculosis drug, cycloserine (Cs) is a broad-spectrum antibiotic introduced in 1952 (5). Cs works on cell wall biosynthesis by inhibiting d-alanine:d-alanine ligase (Ddl) and alanine racemase (Alr), which are involved in pentapeptide core formation (6–8). One advantage of using Cs is that fewer patients with drug-resistant disease have been reported than for some other second-line antituberculosis drugs (9). Thus, Cs was recommended by the World Health Organization (WHO) to be administered to MDR-TB patients due to the lack of cross-resistance with other anti-TB drugs (10).
Few studies have been performed to evaluate the efficacy of Cs-containing regimens in the treatment of MDR- or XDR-TB. Additionally, no predictor of treatment response to Cs-containing regimens had been reported. In this study, we evaluated the in vitro bacteriostatic effect of Cs and also reported our clinical experience with Cs for therapy of MDR/XDR-TB patients in Beijing, China. Additionally, the correlations of Cs treatment outcomes with different risk factors were analyzed. Our study will improve the understanding of use of Cs as a candidate drug to treat drug-resistant TB, and it is the first report on Cs efficacy evaluation from China.
RESULTS
Cs MIC distribution for 104 clinical strains.
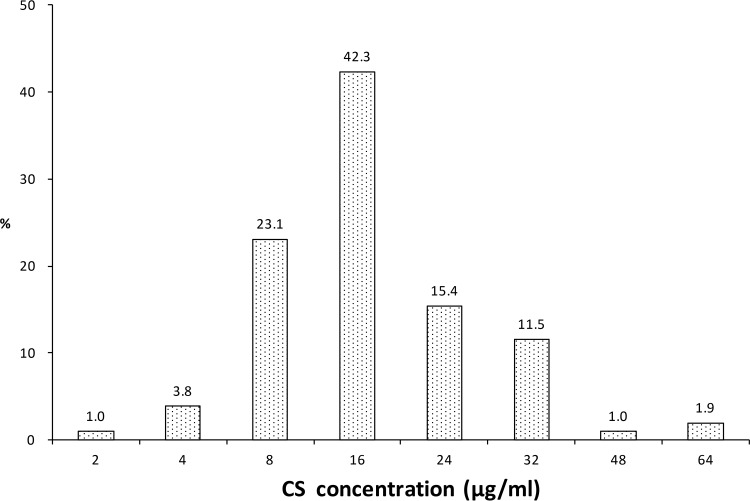
The MIC distribution for 104 clinical Mycobacterium tuberculosis strains is presented in Fig. 1. The Cs MIC50, MIC90, and epidemiological cutoff value (ECOFF) for the tested strains were 16 μg/ml, 32 μg/ml, and 32 μg/ml, respectively. There was no statistically significant difference in the MICs of Cs between the MDR group (n = 29) and the non-MDR group (n = 75) (Mann-Whitney U = 10,420.5, Z = −0.343, P = 0.732). The MICs for the control laboratory strain M. tuberculosis H37Rv in triplicate assays were uniform at 8 μg/ml.
FIG 1.
Cycloserine MIC distribution for 104 M. tuberculosis clinical isolates.
Serum concentration of Cs.
A total of 73 MDR-TB patients who met the requirements were enrolled. Blood was drawn after 1 week of Cs treatment for serum concentration monitoring. Fifty-two patients and 21 patients were initially administered Cs at dosages of 500 mg/day and 750 mg/day, respectively. Seven patients had the Cs dosage adjusted according to the first therapeutic drug monitoring (TDM) outcomes: 2 patients had serum drug concentrations below 20 μg/ml and thus had the dosage increased from 500 mg/day to 750 mg/day, and 5 patients had serum drug concentrations above 35 μg/ml and thus had the dosage decreased from 750 mg/day to 500 mg/day. For these patients, a second blood sample was drawn after the new dosage had been administered for 1 week.
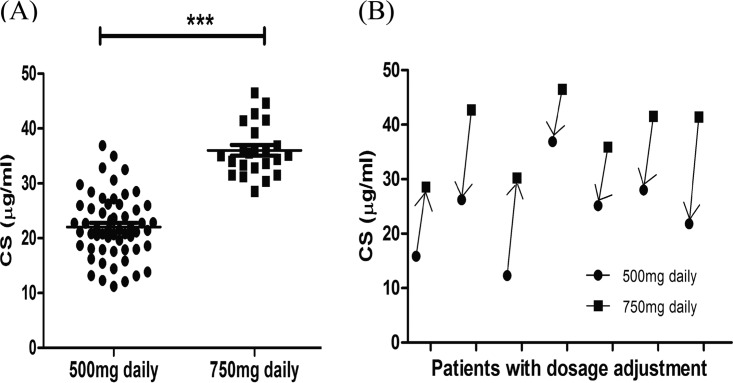
The serum Cs concentration outcomes for the 73 patients are presented in Table 1 and Fig. 2A. The 750-mg/day dosage group had significantly higher serum concentrations than the 500-mg/day dosage group (t = −10.308, P < 0.001). A total of 65.8% (48/73) of the enrolled patients had Cs serum concentrations between 20 and 35 μg/ml, whereas about one-third (18 out of 52) of the 500-mg/day dosage group had serum concentrations lower than 20 μg/ml, and more than half (13 out of 21) of the 750-mg/day dosage group had serum concentrations higher than 35 μg/ml. The altered serum concentrations for the 7 patients who had adjusted dosages are presented in Fig. 2B.
TABLE 1.
Cs peak serum concentrations for patients receiving different dosagesa
| Parameter | Value for patients receiving: |
|
|---|---|---|
| 500 mg/day (n = 57) | 750 mg/day (n = 23) | |
| Serum concn (μg/ml) | ||
| Range | 11.21–36.90 | 28.57–46.51 |
| Mean ± SD | 22.06 ± 5.77 | 36.03 ± 4.68 |
| No. with serum concn (μg/ml): | ||
| <20 | 18 | 0 |
| 20–35 | 38 | 10 |
| >35 | 1 | 13 |
The outcomes before and after the dosage adjustment for the 7 patients with dosage adjustment were all counted.
FIG 2.
Two-hour postdose peak Cs serum concentrations for the enrolled patients. (A) Serum concentration distribution of 80 tests for 73 patients. ***, P < 0.001. (B) Serum concentration changes for the 7 patients with dosage adjustment. Two patients had their dosage increased from 500 mg/day to 750 mg/day, and five patients had their dosage decreased from 750 mg/day to 500 mg/day.
Treatment outcome and toxicity follow-up for patients with baseline isolates.
Among the 73 enrolled patients, isolates were obtained from 27 patients just before enrollment. Among them, 21 were male, and the mean age was 40 years old (range, 17 to 63). Twenty-six out of the 27 patients had been treated with the second-line drugs for more than 1 year before starting the Cs-containing regimens. Nine (33.3%) of the patients had XDR-TB, while 44.4% (12/27) were pre-XDR-TB patients (having MDR-TB plus resistance to either fluoroquinolone or at least one of three injectable second-line drugs), and the other 6 were defined as simple MDR-TB patients (having MDR-TB still sensitive to fluoroquinolone and aminoglycoside). The patients were treated with Cs for a median duration of 14 months (interquartile range [IQR], 6 to 24 months). Eleven (40.7%) of the 27 patients were cured, but 16 failed to be cured. No patient death occurred during the study period. Two patients with the 500-mg/day dosage had mild to moderate anxiety and depression. Table S1 in the supplemental material shows the specific details for each case.
Risk factors influencing treatment outcomes of Cs-containing regimens.
Drug resistance severity and low peak serum concentration (Cmax) were likely to influence the treatment outcomes, but there was no significant difference between different groups, probably because of the small sample size. Notably, we found that Cs susceptibility correlated with clinical treatment outcomes. All the patients infected with M. tuberculosis isolates with MICs of ≥32 μg/ml had unfavorable clinical outcomes (100%, 5/5). Statistical analysis revealed that the percentage of favorable outcomes among patients with a Cmax/MIC ratio of ≥1 was statistically higher than that among patients with a Cmax/MIC ratio of <1 (P = 0.022). The evaluation of different predictors for treatment outcome is summarized in Table 2.
TABLE 2.
Treatment outcome predictors for the enrolled patientsa
| Factor | No. of patients |
Odds ratio (95% confidence interval) | P value | |
|---|---|---|---|---|
| Total | With favorable outcome | |||
| Age (yr) | ||||
| ≤20 | 3 | 0 | NA | 0.491 |
| >20–≤40 | 9 | 4 | 1 | |
| >40–<60 | 14 | 7 | 1.250 (0.233–6.715) | 1.000 |
| ≥60 | 1 | 0 | NA | 1.000 |
| Gender | ||||
| Male | 21 | 7 | 1 | |
| Female | 6 | 4 | 4.000 (0.584–27.411) | 0.187 |
| Drug resistance pattern | ||||
| Simple MDR | 6 | 4 | 1 | |
| Pre-XDR | 12 | 5 | 0.357 (0.046–2.771) | 0.620 |
| XDR | 9 | 2 | 0.143 (0.014–1.444) | 0.136 |
| Dosage (mg/day) | ||||
| 500 | 23 | 8 | 1 | |
| 750 | 4 | 3 | 5.625 (0.500–63.282) | 0.273 |
| MIC (μg/ml) | ||||
| <32 | 22 | 11 | 1 | |
| ≥32 | 5 | 0 | NA | 0.06 |
| Cmax (μg/ml) | ||||
| ≤20 | 11 | 3 | 0.429 (0.081–2.277) | 0.428 |
| >20–<35 | 15 | 7 | 1 | |
| ≥35 | 1 | 1 | NA | 1.00 |
| Cmax/MIC | ||||
| <1 | 15 | 3 | 1 | 0.022 |
| ≥1 | 12 | 8 | 8.000 (1.399–45.756) | |
Cmax, serum concentration after 2 h; NA, not applicable.
DISCUSSION
The in vitro susceptibility of Mycobacterium tuberculosis to many second-line drugs (other than fluoroquinolones and injectable agents) is questionable due to the weak clinical relevance of the drugs (11). For the less frequently used but widely recognized drug Cs, susceptibility testing for treatment of TB has not been well established. Kam et al. (12) suggested a tentative critical concentration for Cs at 30 μg/ml based on the MIC distributions for possible susceptible and possible resistant M. tuberculosis strains, and when they adopted this criterion, they found that 82% of possible resistant strains were misclassified as susceptible ones based on the clinical history judgments (12). In our study, we determined the ECOFF for Cs with a liquid broth method and interpreted the pharmacokinetic data for predicting treatment outcomes.
In China, Cs was approved for infection treatment in May 2014 by the Chinese Food and Drug Administration. Therefore, the M. tuberculosis isolates collected before 2014 had never been exposed to Cs and could be considered wild-type strains. In this study, the MICs of Cs against wild-type strains showed a normal distribution within the range of 2 μg/ml to 64 μg/ml (Fig. 1). The MIC distribution for the wild-type strains showed that the ECOFF was 32 μg/ml for Cs, which indicated that 32 μg/ml could be defined as the tentative critical concentration for Cs. However, the mean peak serum concentration of Cs at the 500-mg/day dosage was 22.06 ± 5.77 μg/ml. The Cs concentrations in the plasma of most patients at this dosage were far below 32 μg/ml; only 4 out of 57 patients surpassed the MIC90. On the other hand, 18 out of 23 patients in the 750-mg/day dosage group had a Cmax above 32 μg/ml. These outcomes indicated that for patients infected with a strain that has an obviously elevated MIC of Cs, the 500-mg/day dosage might not be very effective. ECOFF reflects only the distribution of in vitro MICs for the wild-type strains, and thus the clinical response of the patients and the attainable concentrations in serum and tissue must be considered for setting the critical concentration. Therefore, a tentative concentration set based on ECOFF only is subject to adjustment when more clinical data are available. WHO decreased the critical concentration for Cs susceptibility testing from 40 μg/ml to 30 μg/ml in the new guideline in 2014 (13). Our ECOFF data justify this adjustment, whereas our TDM data suggest an even lower concentration. More studies and more data are needed.
TDM remains a standard clinical practice for determining the appropriate dosage or dosing interval. Recently, dried blood spots have been successfully used for TDM, which makes TDM more convenient and less expensive (14). A previous study acquired peak serum concentrations of Cs between 20 and 35 μg/ml when dosing was at 250 to 500 mg per day (15). In our study, to comply with the recommended dosage of 10 mg/kg, patients were prescribed 500 mg or 750 mg Cs daily depending on whether they were lighter or heavier than 50 kg. Notably, the group with the 750-mg/day dosage obtained noticeably higher serum concentrations than the group given 500 mg/day (t = −10.308, P < 0.001). One-third of the 500-mg/day group had serum concentrations lower than 20 μg/ml, while half of the 750-mg/day group exceeded 35 μg/ml, which highlights the necessity of TDM in clinical practice to ensure Cs efficacy and avoid side effects. Seven of our enrolled patients had their dosages adjusted according to TDM and achieved the peak concentration within the targeted range of 20 to 35 μg/ml (Fig. 2B), which justified the clinical practices. In our study, both of the patients developed psychotic symptoms at the 500-mg/day dosage, and the peak serum concentration in one patient was only 12.12 μg/ml. However, both patients were administered fluoroquinolone simultaneously, and psychotic symptoms are one of its possible side effects as well (16, 17). Although a peak serum concentration lower than 20 μg/ml was a risk factor for unfavorable treatment outcome (odds ratio [OR] = 0.429), our assay did not show a statistically significant difference between groups with Cs concentrations lower than or within the recommend concentrations considering the therapy outcomes (P = 0.428). A recent study by Hung et al. (28) also revealed that 17 of 18 MDR-TB patients were cured with a peak Cs concentration of <20 μg/ml. As MDR-TB treatment involves a combination of multiple drugs, treatment outcome is often not a good indicator to evaluate the efficacy of a single drug. Although our study and others demonstrated that TDM alone could not well predict the efficacy of Cs, the studies enrolled a very small number of patients, which could cause bias.
Since huge MIC variations for Cs were observed among wild-type strains, we took the MIC value for the given patient into consideration. Our study showed that patients with a Cmax/MIC ratio of ≥1 had a significantly higher chance of having favorable treatment outcomes than those with a Cmax/MIC ratio of <1 (P < 0.05), which meant that a drug concentration above the MIC for the infecting bacteria may be a good indicator for favorable treatment outcomes. Stratifying analyses according to drug resistance severity and treatment duration were performed as well (data not shown). For all the stratified groups, the outcomes supported that patients with a Cmax/MIC ratio of ≥1 had a higher chance of having favorable treatment outcomes, although the small number of patients made the conclusions less strong. Because of the low success rate in MDR-TB treatment, side effects, and high cost of the drug, an appropriate indicator will be helpful for clinicians when establishing a regimen containing Cs. Currently, commercial kits such as the Trek Sensititre MycoTB MIC plate (Trek Diagnostic Systems, Cleveland, OH) can provide MIC information for Cs (18–20), which makes the determination of the Cmax/MIC ratio more feasible.
In an early study on MDR-TB treatment, Tahaoglu et al. reported an overall response rate of 77% among 158 MDR-TB patients; 142 of those patients were administered Cs (21). In other studies using Cs, the rate of successful treatment rate was 67.5% (29/43) in Iran (22), and a report from India showed that 71% (10/14) of the MDR-TB patients achieved sputum conversion within 6 months of therapy (23). Compared with other reports, our study showed a much lower favorable treatment rate (40.7%). We attributed those differences to the variations among the patients enrolled. First, our patients had more severe drug resistance patterns, as 33.3% (9/27) and 44.4% (12/27) of them were XDR- or pre-XDR-TB patients. Second, 26 out of the 27 MDR-TB patients with baseline isolates in our study had been treated for MDR-TB for at least 1 year, whereas pretreated MDR-TB patients were always excluded from the randomized, double-blind, placebo-controlled studies (24, 25). In the real world, patients with a previous treatment history for MDR-TB are very common. Thus, evaluation of drug efficacy among those patients was necessary and useful.
Our study has some limitations. First, due to the small number of enrolled patients, our conclusions still need more validation by multiple centers. Second, our study was carried out under very realistic conditions, so the design could not be very optimal, which made our conclusions less strong. For example, due to ethical prohibition and patients' unwillingness, the serum Cs concentration was determined only once for the majority of the patients, which made a detailed pharmacokinetics analysis impossible. We hope our work will provide some insights on the value of Cs for MDR-TB treatment and generate more interest in its intensive study.
Conclusion.
Regimens containing Cs, an oral bacteriostatic second-line anti-TB drug, led to favorable treatment outcomes for some MDR/XDR-TB patients, and the Cmax/MIC ratio might be a good indicator for predication of treatment outcome. In a high percentage of patients administered the recommended Cs dosage of 10 mg/kg, desirable blood concentrations could not be obtained, and adjusting the dosage according to TDM is very necessary in clinical practice. Our study proposes an ECOFF for Cs susceptibility testing at 32 μg/ml.
MATERIALS AND METHODS
Ethics statement.
A prospective study was conducted at Beijing Chest Hospital, Beijing, China. The chemotherapeutic regimens and other patient care activities for the enrolled patients were prescribed independently by the physicians without any influence from the project. The study protocol was approved by the institutional Ethical Review Committee of the hospital. Written informed consent was obtained from each involved patient.
Evaluation of in vitro bacteriostatic effects of Cs against clinical isolates of M. tuberculosis.
A total of 104 clinical isolates of M. tuberculosis, including 29 MDR isolates, were collected from August 2010 to December 2010. Cycloserine powder was purchased from Sigma-Aldrich (St. Louis, MO, USA). To determine the MICs of Cs against the clinical isolates, the microplate alamarBlue assay (MABA) was performed as described previously (26). The tested concentrations were 2 μg/ml, 4 μg/ml, 8 μg/ml, 16 μg/ml, 24 μg/ml, 32 μg/ml, 48 μg/ml, and 64 μg/ml. The MIC for the laboratory strain M. tuberculosis H37Rv (ATCC 27294) was tested in triplicate. Since at the time of collection of all the isolates, Cs had never been used in China previously, all the included strains could be considered wild-type strains. The epidemiological cutoff value (ECOFF), which was defined as the lowest concentration of drug that will inhibit 95% of wild-type strains of M. tuberculosis that have never been exposed to drugs, was determined (27).
Patient enrollment.
MDR-TB patients older than 16 with a history of TB treatment and receiving treatment with a Cs-containing regimen during September 2012 to September 2013 were continuously enrolled. Patients who were allergic to Cs or with a positive human immunodeficiency virus (HIV) test result were excluded from the trial. The daily Cs dosage was targeted at around 10 mg/kg. Patients were administered 250 mg Cs either every 12 h (q12h) or q8h before meals for designated dosages of 500 mg or 750 mg per day (500 mg for patient lighter than 50 kg and 750 mg for patients heavier than 50 kg).
Monitoring of serum concentrations of Cs.
For all the enrolled patients, after administration of Cs for a week, venous blood was drawn 2 h after drug administration in the morning for therapeutic drug monitoring (TDM). Some patients had their dosage adjusted according to the first TDM outcomes; therefore, a second blood sample was drawn for another TDM test. The serum concentration of Cs was determined using high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS).
Briefly, 100 μl of a plasma sample was deproteinized with 200 μl of acetonitrile. The mixture was vortexed for 10 s and centrifuged for 10 min at 12,000 × g, and then 2 μl of the supernatant was injected into the chromatography system. The mobile phase was a mixture of liquids (0.1% formic acid and 5 mM ammonium formate-acetonitrile [95:5]). The multiple reaction monitoring transition was 103.2 to 75.1 (m/z) for Cs. The detection was linear over the range of 0.5 to 50 μg/ml (r = 0.999). Coefficients of variation (CV) for both intra- and interday precision were less than 5%. The limits of quantification and detection of Cs were 0.2 μg/ml and 0.01 μg/ml, respectively.
Evaluation of in vivo efficacy of Cs among MDR-TB patients who had baseline isolates.
Among the enrolled MDR-TB patients, the patients who had newly isolated strains just before enrollment were followed up. MICs of Cs against the recovered strains were tested using the aforementioned method, and the peak Cs serum concentration (Cmax)/MIC ratio was calculated. Clinical evaluation of the patients was conducted at least once a month by prescribing sputum smear and culture, hemogram, and biochemistry examination. Computed tomography of the chest was performed when clinical signs indicated. Inquiries about symptoms of psychosis, including anxiety, depression, and seizure, were made at baseline and then monthly during the Cs therapy. For the treatment response evaluation, favorable outcome and unfavorable outcome were defined according to the recommendation of WHO (10). Patients were classified as cured if they had at least five consecutive negative cultures taken at least 30 days apart during the final 12 months of treatment.
Statistical analysis.
Data are presented as mean and standard deviation for normally distributed data. The differences in peak serum concentrations between the 500-mg and 750-mg dosage groups were analyzed by t test. Fisher's exact test was used to investigate demographic and clinical factors associated with the treatment outcomes. A P value of <0.05 was considered to be statistically significant. Statistical analysis was performed using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA).
Supplementary Material
ACKNOWLEDGMENTS
The strains used in this project were obtained from the Beijing Bio-Bank of Clinical Resources on Tuberculosis (D09050704640000), Beijing Chest Hospital. This study was supported by research funding from the Infectious Diseases Special Project, Ministry of Health of China (2017ZX10201301-004-002), the Natural Science Fund of China (81672065), the Capital Health Research and Development of Special (2016-4-1042), and the Tongzhou District Science and Technology Committee (KJ2017CX07).
We have no conflicts of interest to disclose.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01824-17.
REFERENCES
- 1.Maartens G, Wilkinson RJ. 2007. Tuberculosis. Lancet 370:2030–2043. [DOI] [PubMed] [Google Scholar]
- 2.Zhao Y, Xu S, Wang L, Chin DP, Wang S, Jiang G, Xia H, Zhou Y, Li Q, Ou X, Pang Y, Song Y, Zhao B, Zhang H, He G, Guo J, Wang Y. 2012. National survey of drug-resistant tuberculosis in China. N Engl J Med 366:2161–2170. doi: 10.1056/NEJMoa1108789. [DOI] [PubMed] [Google Scholar]
- 3.Kwon YS, Kim YH, Suh GY, Chung MP, Kim H, Kwon OJ, Choi YS, Kim K, Kim J, Shim YM, Koh WJ. 2008. Treatment outcomes for HIV-uninfected patients with multidrug-resistant and extensively drug-resistant tuberculosis. Clin Infect Dis 47:496–502. doi: 10.1086/590005. [DOI] [PubMed] [Google Scholar]
- 4.Chan ED, Laurel V, Strand MJ, Chan JF, Huynh ML, Goble M, Iseman MD. 2004. Treatment and outcome analysis of 205 patients with multidrug-resistant tuberculosis. Am J Respir Crit Care Med 169:1103–1109. doi: 10.1164/rccm.200308-1159OC. [DOI] [PubMed] [Google Scholar]
- 5.Anonymous. 2008. Cycloserine. Tuberculosis (Edinb) 88:100–101. doi: 10.1016/S1472-9792(08)70007-6. [DOI] [PubMed] [Google Scholar]
- 6.Halouska S, Fenton RJ, Zinniel DK, Marshall DD, Barletta RG, Powers R. 2014. Metabolomics analysis identifies d-alanine-d-alanine ligase as the primary lethal target of d-cycloserine in mycobacteria. J Proteome Res 13:1065–1076. doi: 10.1021/pr4010579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruning JB, Murillo AC, Chacon O, Barletta RG, Sacchettini JC. 2011. Structure of the Mycobacterium tuberculosis d-alanine:d-alanine ligase, a target of the antituberculosis drug d-cycloserine. Antimicrob Agents Chemother 55:291–301. doi: 10.1128/AAC.00558-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Awasthy D, Bharath S, Subbulakshmi V, Sharma U. 2012. Alanine racemase mutants of Mycobacterium tuberculosis require d-alanine for growth and are defective for survival in macrophages and mice. Microbiology 158:319–327. doi: 10.1099/mic.0.054064-0. [DOI] [PubMed] [Google Scholar]
- 9.Hong W, Chen L, Xie J. 2014. Molecular basis underlying Mycobacterium tuberculosis d-cycloserine resistance. Is there a role for ubiquinone and menaquinone metabolic pathways? Expert Opin Ther Targets 18:691–701. doi: 10.1517/14728222.2014.902937. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. 2008. WHO guidelines for programmatic management of MDR-TB. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 11.Kim SJ. 2005. Drug-susceptibility testing in tuberculosis: methods and reliability of results. Eur Respir J 25:564–569. doi: 10.1183/09031936.05.00111304. [DOI] [PubMed] [Google Scholar]
- 12.Kam KM, Sloutsky A, Yip CW, Bulled N, Seung KJ, Zignol M, Espinal M, Kim SJ. 2010. Determination of critical concentrations of second-line anti-tuberculosis drugs with clinical and microbiological relevance. Int J Tuberc Lung Dis 14:282–288. [PubMed] [Google Scholar]
- 13.World Health Organization. 2014. Companion handbook to the WHO guidelines for the programmatic management of drug-resistant tuberculosis. World Health Organization, Geneva, Switzerland. [PubMed] [Google Scholar]
- 14.Sotgiu G, Alffenaar JW, Centis R, D'Ambrosio L, Spanevello A, Piana A, Migliori GB. 2015. Therapeutic drug monitoring: how to improve drug dosage and patient safety in tuberculosis treatment. Int J Infect Dis 32:101–104. doi: 10.1016/j.ijid.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Alsultan A, Peloquin CA. 2014. Therapeutic drug monitoring in the treatment of tuberculosis: an update. Drugs 74:839–854. doi: 10.1007/s40265-014-0222-8. [DOI] [PubMed] [Google Scholar]
- 16.Owens RC Jr, Ambrose PG. 2005. Antimicrobial safety: focus on fluoroquinolones. Clin Infect Dis 41(Suppl 2):S144–S157. doi: 10.1086/428055. [DOI] [PubMed] [Google Scholar]
- 17.Mazhar F, Akram S, Haider N. 2016. Moxifloxacin-induced acute psychosis: a case report with literature review. J Res Pharm Pract 5:294–296. doi: 10.4103/2279-042X.192457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall L, Jude KP, Clark SL, Dionne K, Merson R, Boyer A, Parrish NM, Wengenack NL. 2012. Evaluation of the Sensititre MycoTB plate for susceptibility testing of the Mycobacterium tuberculosis complex against first- and second-line agents. J Clin Microbiol 50:3732–3734. doi: 10.1128/JCM.02048-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee J, Armstrong DT, Ssengooba W, Park JA, Yu Y, Mumbowa F, Namaganda C, Mboowa G, Nakayita G, Armakovitch S, Chien G, Cho SN, Via LE, Barry CE III, Ellner JJ, Alland D, Dorman SE, Joloba ML. 2014. Sensititre MYCOTB MIC plate for testing Mycobacterium tuberculosis susceptibility to first- and second-line drugs. Antimicrob Agents Chemother 58:11–18. doi: 10.1128/AAC.01209-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heysell SK, Pholwat S, Mpagama SG, Pazia SJ, Kumburu H, Ndusilo N, Gratz J, Houpt ER, Kibiki GS. 2015. Sensititre MycoTB plate compared to Bactec MGIT 960 for first- and second-line antituberculosis drug susceptibility testing in Tanzania: a call to operationalize MICs. Antimicrob Agents Chemother 59:7104–7108. doi: 10.1128/AAC.01117-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tahaoglu K, Torun T, Sevim T, Atac G, Kir A, Karasulu L, Ozmen I, Kapakli N. 2001. The treatment of multidrug-resistant tuberculosis in Turkey. N Engl J Med 345:170–174. doi: 10.1056/NEJM200107193450303. [DOI] [PubMed] [Google Scholar]
- 22.Masjedi MR, Tabarsi P, Chitsaz E, Baghaei P, Mirsaeidi M, Amiri MV, Farnia P, Javanmard P, Mansouri D, Velayati AA. 2008. Outcome of treatment of MDR-TB patients with standardised regimens, Iran, 2002-2006. Int J Tuberc Lung Dis 12:750–755. [PubMed] [Google Scholar]
- 23.Prasad R, Verma SK, Sahai S, Kumar S, Jain A. 2006. Efficacy and safety of kanamycin, ethionamide, PAS and cycloserine in multidrug-resistant pulmonary tuberculosis patients. Indian J Chest Dis Allied Sci 48:183–186. [PubMed] [Google Scholar]
- 24.Diacon AH, Pym A, Grobusch MP, de los Rios JM, Gotuzzo E, Vasilyeva I, Leimane V, Andries K, Bakare N, De Marez T, Haxaire-Theeuwes M, Lounis N, Meyvisch P, De Paepe E, van Heeswijk RP, Dannemann B. 2014. Multidrug-resistant tuberculosis and culture conversion with bedaquiline. N Engl J Med 371:723–732. doi: 10.1056/NEJMoa1313865. [DOI] [PubMed] [Google Scholar]
- 25.Van Deun A, Maug AK, Salim MA, Das PK, Sarker MR, Daru P, Rieder HL. 2010. Short, highly effective, and inexpensive standardized treatment of multidrug-resistant tuberculosis. Am J Respir Crit Care Med 182:684–692. doi: 10.1164/rccm.201001-0077OC. [DOI] [PubMed] [Google Scholar]
- 26.Franzblau SG, Witzig RS, McLaughlin JC, Torres P, Madico G, Hernandez A, Degnan MT, Cook MB, Quenzer VK, Ferguson RM, Gilman RH. 1998. Rapid, low-technology MIC determination with clinical Mycobacterium tuberculosis isolates by using the microplate Alamar Blue assay. J Clin Microbiol 36:362–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Angeby KA, Jureen P, Giske CG, Chryssanthou E, Sturegard E, Nordvall M, Johansson AG, Werngren J, Kahlmeter G, Hoffner SE, Schon T. 2010. Wild-type MIC distributions of four fluoroquinolones active against Mycobacterium tuberculosis in relation to current critical concentrations and available pharmacokinetic and pharmacodynamic data. J Antimicrob Chemother 65:946–952. doi: 10.1093/jac/dkq091. [DOI] [PubMed] [Google Scholar]
- 28.Hung WY, Yu MC, Chiang YC, Chang JH, Chiang CY, Chang CC, Chuang HC, Bai KJ. 2014. Serum concentrations of cycloserine and outcome of multidrug-resistant tuberculosis in Northern Taiwan. Int J Tuberc Lung Dis 18:601–606. doi: 10.5588/ijtld.13.0268. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.