ABSTRACT
Vaborbactam is a member of a new class of β-lactamase inhibitors with inhibitory activity against serine carbapenemases (e.g., Klebsiella pneumoniae carbapenemase) that has been developed in combination with meropenem. The pharmacokinetics of the combination was evaluated in 41 subjects with chronic renal impairment in a phase 1, open-label, single-dose study. Subjects were assigned to one of five groups based on renal function: normal (creatinine clearance of ≥90 ml/min), mild (estimated glomerular filtration rate [eGFR] of 60 to 89 ml/min/1.73 m2), moderate (eGFR of 30 to <60), or severe (eGFR of <30) impairment plus end-stage renal disease (ESRD) patients on hemodialysis. Subjects received a single intravenous dose of 1 g of meropenem plus 1 g of vaborbactam by 3-h infusion. The ESRD group received two doses (on and off dialysis) separated by a washout. Pharmacokinetic parameters were estimated by standard noncompartmental methods. For both meropenem and vaborbactam, the area under the concentration-time curve was larger and the elimination half-life was longer with decreasing renal function. Meropenem and vaborbactam total plasma clearance (CLt) rates were similar and decreased with decreasing renal function. Slopes of the linear relationship between eGFR and CLt were similar, indicating a similar proportional reduction in CLt with decreasing renal function. Hemodialysis significantly increased drug clearance of meropenem (mean of 2.21-fold increase in CLt, P < 0.001) and vaborbactam (mean of 5.11-fold increase, P = 0.0235) relative to drug administration off dialysis, consistent with dose recovery rates of 38.3% and 52.9% for meropenem and vaborbactam, respectively, in dialysate. Plasma clearance of meropenem and vaborbactam is reduced with renal impairment, requiring dose adjustment. Hemodialysis removes both drugs. (This study has been registered at ClinicalTrials.gov under identifier NCT02020434.)
KEYWORDS: meropenem-vaborbactam, pharmacokinetics, renal impairment, hemodialysis
INTRODUCTION
The worldwide spread of resistance to antibiotics among Gram-negative bacteria has resulted in a crisis in the treatment of hospital-acquired infections (1). In particular, the recent dissemination of serine carbapenemases (e.g., Klebsiella pneumoniae carbapenemases [KPC]) in Enterobacteriaceae in U.S. hospitals now poses a considerable threat to carbapenems and other members of the β-lactam class of antimicrobial agents (2).
A fixed-combination antibiotic (Vabomere) composed of meropenem and the new β-lactamase inhibitor vaborbactam has been developed for the treatment of patients with severe Gram-negative infections. Vaborbactam is a newly discovered β-lactamase inhibitor that has activity against serine carbapenemases, particularly KPC, that are the major cause of carbapenem-resistant Enterobacteriaceae (3). Previous work has demonstrated the safety, tolerability, and pharmacokinetic profile of vaborbactam alone and in combination with meropenem in healthy adult volunteers (4–6), revealing that the new β-lactamase inhibitor's pharmacokinetic profile is similar to that of meropenem (7). The purpose of this phase 1 study was to assess the safety, tolerability, and pharmacokinetics of intravenous meropenem-vaborbactam administered to adult subjects with renal impairment and subjects with end-stage renal disease (ESRD) receiving hemodialysis therapy compared to results in subjects with normal renal function.
RESULTS
Demographics.
Table 1 summarizes baseline subject demographics. The majority of subjects were white and male. Subjects' ages ranged from 43 to 73 years.
TABLE 1.
Baseline demographics
| Parametera | Value for the parameter by renal function groupb |
|||||
|---|---|---|---|---|---|---|
| Normal (n = 8) | Mild (n = 8) | Moderate (n = 8) | Severe (n = 8) | ESRD (n = 9) | Total (n = 41) | |
| Sex (no. [%]) | ||||||
| Female | 4 (50) | 4 (50) | 4 (50) | 3 (37.5) | 15 (36.6) | |
| Male | 4 (50) | 4 (50) | 4 (50) | 5 (62.5) | 9 (100) | 26 (63.4) |
| Race (no. [%]) | ||||||
| White | 7 (87.5) | 5 (62.5) | 5 (62.5) | 2 (25.0) | 3 (33.3) | 22 (53.7) |
| Black or African American | 3 (37.5) | 2 (25.0) | 5 (62.5) | 5 (55.6) | 15 (36.6) | |
| Native American or Alaska Native | 1 (12.5) | 1 (11.1) | 2 (4.9) | |||
| Other | 1 (12.5) | 1 (12.5) | 2 (4.9) | |||
| Ethnicity (no. [%]) | ||||||
| Hispanic or Latino | 1 (12.5) | 1 (12.5) | 1 (12.5) | 2 (22.2) | 5 (12.2) | |
| Not Hispanic or Latino | 7 (87.5) | 7 (87.5) | 7 (87.5) | 8 (100) | 7 (77.8) | 36 (87.8) |
| Age (yr) | ||||||
| Mean (SD) | 56.8 (3.4) | 53.4 (8.9) | 62.1 (10.2) | 57.6 (6.4) | 53.8 (10.5) | 56.7 (8.6) |
| Median | 57.0 | 53.5 | 65.0 | 59.0 | 50.0 | 56.0 |
| Range | 52–62 | 44–67 | 45–73 | 49–69 | 43–73 | 43–73 |
| Height (cm) | ||||||
| Mean (SD) | 169.6 (9.1) | 172.9 (9.4) | 170.9 (8.1) | 174.4 (9.6) | 177.8 (4.6) | 173.2 (8.4) |
| Median | 166.5 | 173.7 | 169.8 | 178.5 | 176.7 | 174.0 |
| Range | 160–187 | 156.3–189.6 | 161.3–183.4 | 159.7–186.5 | 172–186 | 156.3–189.6 |
| Weight (kg) | ||||||
| Mean (SD) | 91.6 (11.0) | 99.4 (22.1) | 89.2 (21.4) | 94.2 (19.4) | 106.0 (12.9) | 96.4 (18.0) |
| Median | 91.9 | 98.9 | 87.5 | 85.7 | 106.9 | 94.8 |
| Range | 77.5–107.8 | 67.5–142.6 | 61.1–122.7 | 74.1–125.7 | 85.6–121.6 | 61.1–142.6 |
| BMI (kg/m2) | ||||||
| Mean (SD) | 31.8 (1.8) | 33.1 (5.5) | 30.4 (5.9) | 31.1 (6.7) | 33.6 (4.2) | 32.0 (5.0) |
| Median | 31.0 | 33.8 | 29.8 | 28.0 | 34.1 | 31.2 |
| Range | 30.3–34.7 | 21.5–39.7 | 22.7–40.7 | 26.2–43.8 | 28.0–40.1 | 21.5–43.8 |
| eGFR (ml/min/m2) | ||||||
| Mean (SD) | 99.6 (23.4) | 75.3 (6.2) | 44.1 (8.2) | 15.1 (4.8) | 7.2 (2.4) | 47.3 (37.3) |
| Median | 95.5 | 77.0 | 43.0 | 14.5 | 7.0 | 42.0 |
| Range | 66–141 | 67–83 | 32–56 | 10–25 | 5–13 | 5–141 |
BMI, body mass index; eGFR, estimated glomerular filtration rate. eGFR was calculated using the modification of diet in renal disease (MDRD) equation.
Assignment to renal function groups was based on eGFR values at screening; baseline eGFR values may differ from screening values. ESRD, end-stage renal disease.
Pharmacokinetics.
Examination of the concentration-time profiles across renal function groups revealed that exposure of both meropenem and vaborbactam increased with decreasing renal function (Fig. 1A and B) due to the fact that plasma concentrations of both drugs declined more slowly with decreasing renal function. In group 5 (ESRD), plasma concentrations of both meropenem and vaborbactam declined more rapidly during the on-dialysis session than during the off-dialysis session.
FIG 1.
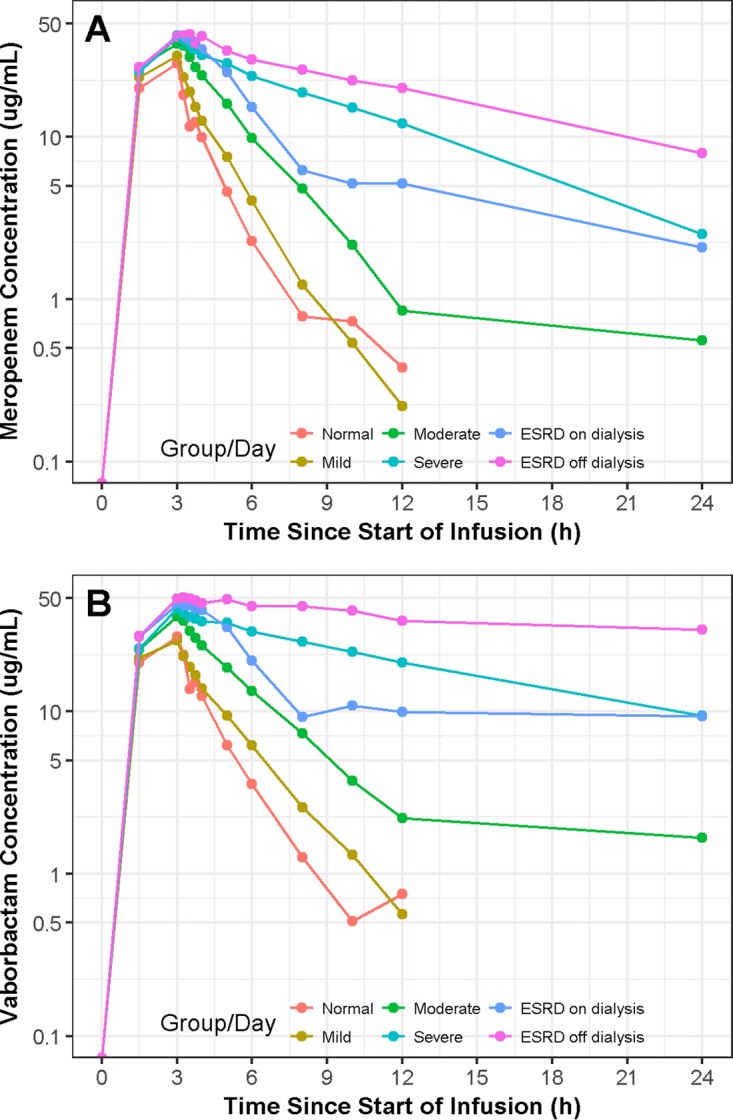
Plots of median meropenem (A) and vaborbactam (B) concentration-time profiles, stratified by renal impairment group and timing relative to dialysis (for end-stage renal disease subjects).
Meropenem and vaborbactam pharmacokinetic parameters are shown in Table 2, summarized by study group. Given that both meropenem and vaborbactam are cleared by the kidneys, consistent differences in all pharmacokinetic parameters were observed in the different renal function groups. For both meropenem and vaborbactam, the area under the plasma concentration time curve (plasma AUC) was larger and the elimination half-life (t1/2) was longer with decreasing renal function. For meropenem, the mean AUC from time zero to infinity (AUC0–inf) increased from a low of 87.1 μg · h/ml in subjects with normal renal function to 112 μg · h/ml in subjects with mild impairment, 181 μg · h/ml in subjects with moderate renal impairment, 397 μg · h/ml in subjects with severe impairment, and 629 μg · h/ml in subjects with ESRD in between dialysis sessions (off dialysis). The trend was similar for vaborbactam, but the magnitude of increase in AUC0–inf was more pronounced as renal function decreased (from 99.4 μg · h/ml in subjects with normal renal function to 781 μg · h/ml in subjects with severe renal impairment and 5,220 μg · h/ml in subjects with ESRD during the off-dialysis period).
TABLE 2.
Pharmacokinetic parameters (mean ± SD) of meropenem and vaborbactam stratified by groupa
| Drug and renal study group | Cmax (μg/ml) | AUC0–inf (μg · h/ml) | AUC0–t (μg · h/ml) | t1/2 (h) | CLNR (liters/h) | CLr (liters/h) | CLt (liters/h) | Vss (liters) |
|---|---|---|---|---|---|---|---|---|
| Meropenem | ||||||||
| Normal (n = 8) | 27.5 ± 7.03 | 87.1 ± 26.7 | 86.4 ± 26.6 | 1.30 ± 0.254 | 4.79 ± 2.18 | 7.69 ± 2.33 | 12.5 ± 3.83 | 19.2 ± 2.69 |
| Mild (n = 8) | 32.7 ± 9.1 | 112 ± 40.6 | 111 ± 40.1 | 1.43 ± 0.179 | 4.11 ± 1.30 | 5.55 ± 2.06 | 9.66 ± 2.42 | 16.5 ± 3.22 |
| Moderate (n = 8) | 40.5 ± 6.27 | 181 ± 59.4 | 177 ± 57.2 | 2.19 ± 0.867 | 2.71 ± 0.811 | 3.34 ± 1.19 | 6.05 ± 1.83 | 16.6 ± 2.21 |
| Severe (n = 8) | 45.5 ± 13.0 | 397 ± 98.0 | 362 ± 83.3 | 6.10 ± 2.59 | 1.69 ± 0.376 | 0.964 ± 0.375 | 2.65 ± 0.606 | 21.6 ± 6.69 |
| ESRD on dialysis (n = 9) | 44.7 ± 8.40 | 280 ± 58.7 | 274 ± 56.8 | 9.33 ± 1.94 | NA | NA | 3.72 ± 0.764 | 30.0 ± 7.18 |
| ESRD off dialysis (n = 8) | 47.7 ± 11.7 | 629 ± 206 | 606 ± 196 | 9.45 ± 1.75 | NA | NA | 1.73 ± 0.512 | 22.2 ± 6.33 |
| Vaborbactam | ||||||||
| Normal (n = 8) | 27.8 ± 6.34 | 99.4 ± 33.0 | 98.0 ± 32.2 | 1.65 ± 0.352 | 0.554 ± 1.69 | 10.5 ± 2.77 | 11.1 ± 3.60 | 20.5 ± 3.53 |
| Mild (n = 8) | 30.1 ± 8.31 | 116 ± 35.9 | 114 ± 34.5 | 1.88 ± 0.274 | 1.67 ± 1.52 | 7.51 ± 1.99 | 9.17 ± 1.98 | 20.8 ± 3.73 |
| Moderate (n = 8) | 42.4 ± 7.45 | 238 ± 108 | 229 ± 99.9 | 3.45 ± 1.71 | 0.642 ± 0.402 | 4.25 ± 1.66 | 4.89 ± 1.78 | 18.8 ± 3.02 |
| Severe (n = 8) | 46.4 ± 12.3 | 781 ± 279 | 532 ± 110 | 13.5 ± 8.71 | 0.288 ± 0.271 | 1.13 ± 0.505 | 1.42 ± 0.465 | 23.3 ± 7.24 |
| ESRD on dialysis (n = 9) | 50.1 ± 10.2 | 1,100 ± 539 | 533 ± 124 | 55.2 ± 33.6 | NA | NA | 1.22 ± 0.799 | 59.1 ± 16.8 |
| ESRD off dialysis (n = 8) | 56.5 ± 13.9 | 5,220 ± 4,620 | 1,640 ± 567 | 79.3 ± 76.2 | NA | NA | 0.405 ± 0.329 | 22.2 ± 5.47 |
Values are means ± standard deviations. Cmax, maximum observed plasma concentration; AUC0–inf, area under the plasma concentration-time curve from time zero to infinity; AUC0–t, area under the plasma concentration-time curve from time zero to the last quantifiable concentration in plasma; t1/2, elimination half-life; CLNR, nonrenal clearance; CLr, renal clearance; CLt, total plasma clearance; Vss, steady-state volume of distribution; ESRD, end-stage renal disease. NA, not available.
The mean steady-state volume of distribution (Vss) values for meropenem and vaborbactam were similar and showed a trend for increased values with decreasing renal function (Table 2). For meropenem, the mean Vss increased from 16.5 liters in subjects with mild impairment to 21.6 liters in subjects with severe impairment and 30.0 liters in subjects with ESRD (on dialysis). For vaborbactam, the increase in Vss was more pronounced, increasing from 20.8 liters in subjects with mild impairment to 59.1 liters in subjects with ESRD (on dialysis). For both meropenem and vaborbactam, the amount of drug excreted via the urine over the 48-h sampling period decreased with decreasing renal function (Tables 3 and 4, respectively).
TABLE 3.
Median values for urine pharmacokinetic parameters for meropenem, stratified by groupa
| Renal study group | Median value for meropenem in urine (range) |
||
|---|---|---|---|
| Amt excreted over 48 h (mg) | % of dose excreted over 48 h | Renal clearance (liters/h) | |
| Normal (n = 8) | 647 (504–727) | 64.7 (50.4–72.7) | 7.19 (5.03–11.9) |
| Mild (n = 8) | 549 (438–724) | 54.9 (43.8–72.4) | 5.37 (2.58–9.58) |
| Moderate (n = 8) | 523 (491–651) | 52.3 (49.1–65.1) | 3.47 (1.90–5.00) |
| Severe (n = 8) | 339 (248–516) | 33.9 (24.8–51.6) | 0.876 (0.569–1.66) |
TABLE 4.
Median values for urine pharmacokinetic parameters for vaborbactam, stratified by group
| Renal study group | Median value for vaborbactam in urine (range) |
||
|---|---|---|---|
| Amt excreted over 48 h (mg) | % of dose excreted over 48 h | Renal clearance (liters/h) | |
| Normal (n = 8) | 990 (760–1,140) | 99.0 (76.0–114) | 10.5 (6.64–14.5) |
| Mild (n = 8) | 856 (642–1,000) | 85.6 (64.2–100) | 7.07 (4.34–10.3) |
| Moderate (n = 8) | 845 (758–975) | 84.5 (75.8–97.5) | 5.21 (2.04–5.97) |
| Severe (n = 8) | 743 (372–941) | 74.3 (37.2–94.1) | 0.978 (0.543–1.98) |
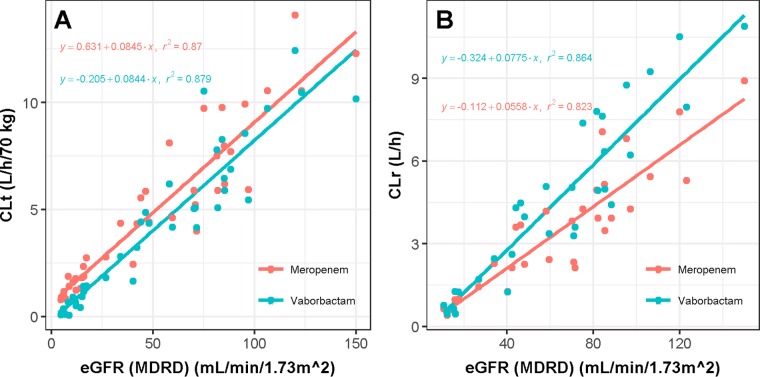
For both compounds, the estimated glomerular filtration rate (eGFR) explained significant portions of the interindividual variability in total plasma clearance (CLt) and renal clearance (CLr) as the r2 values for the relationships were universally above 0.85. The slopes of the regression for eGFR versus CLt were nearly identical (Fig. 2A). The slope of the regression between vaborbactam CLr and eGFR was steeper than that for the relationship between meropenem CLr and eGFR (Fig. 2B), likely because vaborbactam's nonrenal clearance (CLNR) is very low, resulting in a higher correlation between eGFR and CLr.
FIG 2.
Relationship between estimated glomerular filtration rate (eGFR) and total plasma clearance (CLt) (A) and renal clearance (CLr) (B) of meropenem and vaborbactam. Linear regression analyses were conducted separately for the two drugs. Data are only included from groups 1 through 4 and group 5 on dialysis.
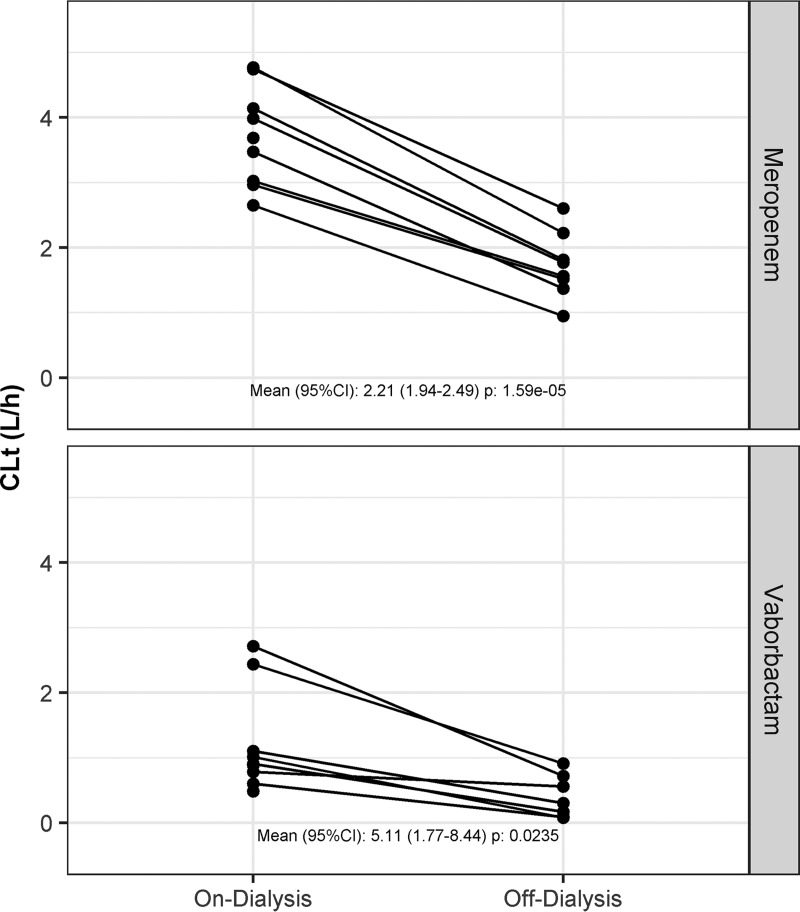
In patients with ESRD (group 5) undergoing hemodialysis, increased drug clearance from a dialysis session is illustrated in Fig. 3. Hemodialysis performed immediately following drug administration (on dialysis) significantly increased drug clearance of both meropenem (mean of 2.21-fold increase in CLt, P < 0.001) and vaborbactam (mean of 5.11-fold increase, P = 0.0235) relative to the level with drug administration after the completion of dialysis (off dialysis). The increase in clearance with dialysis results in a decrease in meropenem and vaborbactam exposure as the AUC estimates from the on-dialysis period were substantially lower than those from the off-dialysis period (Table 2). The differences were most pronounced for vaborbactam; the mean AUC0–inf was nearly 5 times higher off dialysis than the level on dialysis. For meropenem, the mean AUC0–inf was 2.24 times higher off dialysis than that on dialysis. The median percentages of the dose recovered in dialysate during the dialysis session were 38.3% and 52.9% for meropenem and vaborbactam, respectively.
FIG 3.
Effect of hemodialysis on plasma clearance (CLt) of meropenem and vaborbactam patients with ESRD. Values in the figure are on-dialysis/off-dialysis ratios of CLt values. P values were generated using a single-sample t test of the ratio (on-dialysis/off-dialysis). Data are from group 5 only.
Safety.
Table 5 summarizes the treatment-emergent adverse events (TEAEs) by system organ class. For the full study population, 14 subjects (34.1%) reported a total of 20 TEAEs during conduct of the study. The most frequently reported TEAEs were related to diarrhea, headache, abdominal pain, and contact dermatitis. All TEAEs were mild in severity except one moderate TEAE (abdominal pain) and two severe TEAEs (one metastatic prostate cancer and one hemorrhagic diarrhea). Eight of the 20 TEAEs reported in the study were either “possibly” or “probably” related to study treatment and were reported by seven subjects (17.1%). Only one TEAE (metastatic prostate cancer) was ongoing at study completion.
TABLE 5.
Treatment-emergent adverse events (TEAEs)
| System organ class and preferred term | Value for the parameter by renal function group (no. of subjects [%]) |
||||||
|---|---|---|---|---|---|---|---|
| Mild (n = 8) | Moderate (n = 8) | Severe (n = 8) | ESRDb |
Normal (n = 8) | Total (n = 41) | ||
| On dialysis (n = 9) | Off dialysis (n = 8) | ||||||
| Total no. of subjects with any TEAEa | 2 (25.0) | 3 (37.5) | 1 (12.5) | 2 (22.5) | 5 (62.5) | 2 (25.0) | 14 (34.1) |
| Gastrointestinal disorders | 1 (12.5) | 2 (25.0) | 1 (12.5) | 0 | 3 (37.5) | 0 | 7 (17.1) |
| Abdominal pain | 0 | 1 (12.5) | 0 | 0 | 1 (12.5) | 0 | 2 (4.9) |
| Constipation | 0 | 1 (12.5) | 0 | 0 | 0 | 0 | 1 (2.4) |
| Diarrhea | 1 (12.5) | 0 | 1 (12.5) | 0 | 1 (12.5) | 0 | 3 (7.3) |
| Diarrhea hemorrhagic | 0 | 0 | 0 | 0 | 1 (12.5) | 0 | 1 (2.4) |
| Vomiting | 0 | 0 | 0 | 0 | 1 (12.5) | 0 | 1 (2.4) |
| Infections and infestations | 0 | 1 (12.5) | 0 | 0 | 0 | 0 | 1 (2.4) |
| Rhinitis | 0 | 1 (12.5) | 0 | 0 | 0 | 0 | 1 (2.4) |
| Injury, poisoning, and procedural complications | 1 (12.5) | 0 | 0 | 0 | 1 (12.5) | 0 | 2 (4.9) |
| Catheter site phlebitis | 1 (12.5) | 0 | 0 | 0 | 0 | 0 | 1 (2.4) |
| Skin injury | 0 | 0 | 0 | 0 | 1 (12.5) | 0 | 1 (2.4) |
| Musculoskeletal and connective tissue disorders | 0 | 0 | 0 | 1 (11.1) | 0 | 0 | 1 (2.4) |
| Muscle spasms | 0 | 0 | 0 | 1 (11.1) | 0 | 0 | 1 (2.4) |
| Neoplasms benign, malignant and unspecified (including cysts and polyps) | 0 | 0 | 0 | 1 (11.1) | 0 | 0 | 1 (2.4) |
| Prostate cancer metastatic | 0 | 0 | 0 | 1 (11.1) | 0 | 0 | 1 (2.4) |
| Nervous system disorders | 0 | 1 (12.5) | 0 | 1 (11.1) | 2 (25.0) | 0 | 3 (7.3) |
| Headache | 0 | 1 (12.5) | 0 | 1 (11.1) | 1 (12.5) | 0 | 2 (4.9) |
| Paresthesia | 0 | 0 | 0 | 0 | 1 (12.5) | 0 | 1 (2.4) |
| Respiratory, thoracic, and mediastinal disorders | 1 (12.5) | 0 | 0 | 0 | 0 | 0 | 1 (2.4) |
| Sinus congestion | 1 (12.5) | 0 | 0 | 0 | 0 | 0 | 1 (2.4) |
| Skin and subcutaneous tissue disorders | 0 | 0 | 0 | 0 | 0 | 2 (25.0) | 2 (4.9) |
| Dermatitis contact | 0 | 0 | 0 | 0 | 0 | 2 (25.0) | 2 (4.9) |
In the total summary, each subject was counted only once in the category of maximum reported intensity and only once within the ESRD group if an AE occurred during the on- and off-dialysis periods.
ESRD, end-stage renal disease.
There was no evidence of increasing adverse event (AE) incidence or severity with decreased renal function. However, in subjects with ESRD (group 5), a greater number of AEs was observed when meropenem-vaborbactam was administered off dialysis than when meropenem-vaborbactam was administered on dialysis (62.5% versus 22.5%). The AEs occurring during the off-dialysis period were mild in severity except for the single event of hemorrhagic diarrhea, which was classified as severe. Generally, the type and severity of the AEs occurring in the ESRD group were similar to those AEs observed in the other renal function groups.
Two serious adverse events (SAEs) were reported (one metastatic prostate cancer and one hemorrhagic diarrhea), and the event of metastatic prostate cancer resulted in the subject not receiving the second dose of drug in group 5. The event of metastatic prostate cancer was not related to the study drug, and the hemorrhagic diarrhea was possibly related. No AEs resulted in death. There were no clinically significant trends in 12-lead electrocardiogram (ECG) data, vital signs data, clinical laboratory results, or physical examination data.
DISCUSSION
This was a phase 1, open-label, single-dose study to assess the safety, tolerability, and pharmacokinetic profile of meropenem and vaborbactam given in combination as a 3-h intravenous infusion to adults with various degrees of renal impairment, including those with ESRD receiving hemodialysis therapy. Forty-one subjects were enrolled (8 with mild renal impairment, 8 with moderate renal impairment, 8 with severe renal impairment, 9 with ESRD, and 8 with normal renal function), and 40 subjects completed study participation according to the protocol.
The plasma clearance of meropenem and vaborbactam decreased with decreasing renal function. The slopes of the relationship between eGFR and meropenem or vaborbactam plasma clearance were similar, indicating a similar proportional reduction in clearance with decreasing renal function. These data suggest that the proportional dose reduction in subjects with renal impairment should be similar for both meropenem and vaborbactam. However, the magnitude of the decrease in plasma clearance was more pronounced with vaborbactam than with meropenem as renal function decreased, suggesting that vaborbactam may accumulate to a greater extent than meropenem with repeated dosing. Subjects with ESRD undergoing a hemodialysis session showed that plasma clearance of both drugs was increased compared to plasma clearance off dialysis, indicating that both meropenem and vaborbactam are removed by hemodialysis. Removal of both drugs during hemodialysis was shown by recovery of 38.3% and 52.9% of a dose of meropenem and vaborbactam, respectively, in dialysate during a dialysis session. The half-life estimates provided for ESRD patients should be interpreted with caution as they may not be accurate due to the sampling scheme.
A single, 3-h intravenous infusion of 1 g of meropenem plus 1 g of vaborbactam in combination was safe and well tolerated in subjects with normal, mild, moderate, and severe renal impairment, with no evidence of increasing incidence or severity of AEs with declining renal function. In ESRD subjects, the same doses of meropenem and vaborbactam were safe and well tolerated whether administered before or after dialysis; however, a higher number of AEs was observed when meropenem-vaborbactam was administered off dialysis, perhaps due to a higher concentration of both drugs during this session. Overall, the average subject bodyweight across all study groups was considered obese (body mass index [BMI] of >30); however, these subjects are representative of the renal impairment population.
Overall, these data demonstrate the pharmacokinetics, safety, and tolerability of meropenem-vaborbactam following a single dose in patients with various degrees of renal impairment as well as the removal of drug during hemodialysis. These data, along with data from population pharmacokinetic studies in infected patients (8) and Monte Carlo simulation (9), were used to generate dosing recommendations in patients with renal impairment (10).
MATERIALS AND METHODS
Study design.
Plasma and urine pharmacokinetics of meropenem and vaborbactam were assessed in a phase 1, open-label, single-dose study of adult subjects with various degrees of renal impairment (ClinicalTrials.gov identifier NCT02020434). The study was conducted at two DaVita Clinical Research Centers (Lakewood, CO, and Minneapolis, MN). The first subject was screened on 27 January 2014, and the last follow-up occurred on 3 September 2014. Based on previous safety and pharmacokinetic data (7) and the meropenem package insert (11), a single dose of 1 g of meropenem was chosen for this study. A single dose of 1 g was chosen for vaborbactam based on results from previous safety and pharmacokinetic data (4).
A total of 41 subjects were enrolled and formed a total of five study groups based on renal function. Renal function was determined at screening using the eGFR calculated using the modification of diet in renal disease (MDRD) equation (12). Groups 1, 2, and 3 included subjects with mild, moderate, and severe renal impairment, respectively. Group 4 included healthy volunteers with normal renal function that were recruited to be matched to combined groups 1, 2, and 3 based on age (±10 years), sex, and body mass index (BMI) (±20%). The final group consisted of subjects with ESRD receiving hemodialysis therapy three times a week for at least 3 months prior to day 1 of the study who were not matched to the other groups. The total duration of participation in the study for each subject (excluding the screening period) was approximately 7 days.
Both males and females of non-childbearing potential were eligible for inclusion in the study if they were 18 through 80 years of age, inclusive, at the time of screening. Other key inclusion criteria for the study were that subjects had a BMI within the range of 18.5 to 45 kg/m2, sufficient peripheral vascular access for blood collection, and a sitting pulse rate within the range of 50 to 110 beats per min at screening. Study subjects also needed to have negative test results for hepatitis B surface antigen, anti-hepatitis C virus antibody, and anti-HIV antibody. Key exclusion criteria were the following: unstable or new medical conditions in the 3 months prior to day 1; hypersensitivity to β-lactam antibiotics; history of clinically significant seizures, head injury, or meningitis; current evidence or history of malignancy in the 2 years prior to the day before first dosing; previous meropenem-vaborbactam treatment; a heart rate correction using Fridericia's formula (QTcF) of >500 ms or history of prolonged QT syndrome at screening or during the day before first dosing. In addition to these criteria, all subjects prior to undergoing any screening or study activities were required to sign an informed consent form that was approved by a regional institutional review board.
After informed consent was obtained, screening of study subjects was conducted. At the screening visit, a medical history, physical examination, clinical laboratory tests, and electrocardiogram (ECG) were conducted, and renal function was determined. Subjects who passed the screening procedures and were determined to be eligible for the study were assigned to study groups based on renal function. Mild renal impairment was defined as an eGFR of 60 to 89 ml/min/1.73 m2 while moderate renal impairment was defined as an eGFR of 30 to <60 ml/min/1.73 m2, and severe renal impairment was defined as an eGFR of <30 ml/min/1.73 m2. Normal renal function was defined as creatinine clearance, using Cockcroft-Gault (13), of ≥90 ml/min.
Enrolled study subjects who met all inclusion criteria were admitted to the research clinic the day before the first administration of study drug. On day 1 of the study, a single intravenous dose of 1 g of meropenem plus 1 g of vaborbactam was infused over 3 h for all subjects in the renal impairment and normal renal function groups (groups 1 through 4). Subjects were confined in the clinic until completion of postdose procedures on day 3. Study subjects received a follow-up telephone call 2 to 4 days after discharge from the clinic.
In order to determine the impact of hemodialysis therapy on the pharmacokinetics of meropenem and vaborbactam, subjects with ESRD (group 5) were administered the study drug during two separate periods. During the on-dialysis period, study drug administration occurred prior to dialysis on day 1, and subjects remained confined at the clinic until completion of all study assessments on day 3. During the off-dialysis period, dialysis occurred prior to study drug administration on day 1, and subjects remained confined at the clinic until completion of all study assessments on day 3. A washout period of 6 to 14 days occurred between these two drug administration periods. The on- and off-dialysis periods did not have to occur in a specific sequence. The dialysis duration in each period was 5 h, and the majority of subjects were using a Fresenius Optiflux dialyzer, with the same type of dialyzer used for each session. The range of average blood flow was 400 to 600 ml/min, and the range of average dialysate flow rates was 600 to 800 ml/min. As with groups 1 through 4, study subjects received a follow-up telephone call 2 to 4 days after discharge from the clinic.
Western Institutional Review Boards (Puyallup, Washington, USA) reviewed and approved this study to be conducted at both DaVita Clinical Research clinical sites in Minneapolis, MN, and Lakewood, CO.
Safety.
Safety was assessed at multiple time points from check-in (1 day prior to study drug administration on day 1) through day 3. This was accomplished through monitoring of vital signs, ECGs, and clinical laboratory tests, including hematology, coagulation, serum chemistry, and urinalysis tests. Additionally, any AEs that occurred during the study period were noted. Specific inquiries were used to assess the AE status of study subjects during assessments on days 1 through 3, and subjects were contacted via telephone 2 to 4 days after discharge on day 3 to determine the status of any ongoing AEs. All AEs considered possibly or probably related to the study drug were followed until the subject was deemed stable or the AE was resolved. Each AE reported was graded according to the current National Institutes of Health National Cancer Institute common terminology criteria for adverse events toxicity grading scale, a 5-point severity scale (14), and all AEs were coded using MedDRA, version 16.1. The number and severity of TEAEs resulting from administration of meropenem-vaborbactam were evaluated and compared across renal impairment groups.
The investigator and the sponsor reviewed all pertinent blinded safety data in an ongoing fashion. The protocol stated that if two or more subjects experienced a drug-related toxicity grade of ≥3 in any group, that group would be stopped, pending safety review and final dosing decision per the investigator and the sponsor. All groups completed the study as planned. One subject in the ESRD group (group 5) discontinued due to a TEAE of metastatic prostate cancer.
Pharmacokinetic samples and analyses.
For all subjects, blood samples were collected for pharmacokinetic assessments at the following time points: predose and at 1.5 (mid-infusion), 3 (end of infusion), 3.25, 3.5, 3.75, 4, 5, 6, 8, 10, 12, and 24 h after dosing. After collection of samples, blood was centrifuged, and plasma was collected. Plasma was diluted 1:1 with 3-(-N-morpholino) propanesulfonic acid (MOPS) buffer to stabilize meropenem, thoroughly mixed, and frozen. For all subjects except those undergoing hemodialysis therapy, urine samples for pharmacokinetic assessment were collected predose and during the following intervals: 0 to 4, 4 to 8, 8 to 12, 12 to 24, and 24 to 48 h after dosing. On day 1, the predose urine sample was collected prior to dosing. Urine sample volumes were recorded for each collection period, and an aliquot was removed and diluted 1:1 with MOPS buffer, mixed, and frozen. For subjects undergoing hemodialysis, dialysate samples were collected hourly during the 5-h dialysis period, and the amount of dialysate was recorded. An aliquot was removed and diluted 1:1 with MOPS buffer, thoroughly mixed, and frozen. Plasma, urine, and dialysate samples were assayed for meropenem or vaborbactam concentrations using validated procedures and methods.
The pharmacokinetic parameters of interest were maximum concentration (Cmax), AUC from time zero to the time of the last quantifiable concentration in plasma (AUC0–t), and AUC0–inf. Other pharmacokinetic measures were the time at which the maximum concentration occurs (Tmax), Vss, and t1/2. Urine pharmacokinetic parameters such as amount excreted (Ae), percent dose excreted (fe), and CLr were calculated from urinary excretion data. Renal clearance was calculated using the following equation: CLr = Ae0–t/AUC0–t.
Bioanalytical procedures for determination of meropenem and vaborbactam concentrations.
Concentrations of meropenem and vaborbactam in plasma, urine, and dialysate were measured by high-performance liquid chromatography–tandem mass spectrometry at MicroConstants, Inc. (San Diego, CA, USA).
The calibration range of the plasma assay for meropenem and vaborbactam was linear (r2 of ≥0.999) from 0.2 to 100 μg/ml. In total, 651 unique samples were analyzed in 11 analytical runs, and all met acceptance criteria for standard curve and quality control (QC) samples. The accuracy of the method was determined by comparing the mean measured concentrations with theoretical concentrations of each analyte in the QC samples. The deviations of the mean from theoretical values did not exceed ±1.67% and ±1.17% for meropenem and vaborbactam, respectively. The precision was determined from the percent coefficient of variation (%CV) of the QC sample replicates at each concentration level. The %CVs for meropenem and vaborbactam ranged from 4.61% to 5.81% and 3.66% to 6.26%, respectively.
Statistical analyses.
Statistical analyses (including summary statistics) were conducted using qualified installations of SAS (version 9.2 or higher); some graphical presentations of data were generated using R, version 3.1.2. Pharmacokinetic parameters were calculated from the individual plasma concentrations by noncompartmental analysis using a validated installation of Phoenix WinNonlin, version 6.3, for the actual, rather than the scheduled, times of sample collection. For semilog plots and AUC0–t, all postdose concentration values below the lower limit of quantitation were treated as missing. Summary statistics were tabulated by group and period (where applicable) for the pharmacokinetic parameters.
The relationship between drug clearance and renal function was quantified using linear regression. For the linear regression analyses, the dependent variables were weight-normalized CLr (groups 1 to 4 only) and weight-normalized CLt (groups 1 to 4 and group 5, off dialysis). Meropenem and vaborbactam parameters were analyzed separately. Using the data from group 5 alone, the impact of dialysis on the pharmacokinetics of meropenem and vaborbactam was quantified using the ratio of the CLt during the on-dialysis period to the CLt during the off-dialysis period. The probability that the sample mean ratio differed significant from unity (1.0) was tested using a single-sample t test.
ACKNOWLEDGMENTS
This project has been funded in part by The Medicines Company and the Department of Health and Human Services, Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority (BARDA), under contract number HHSO100201400002C with Rempex Pharmaceuticals, a wholly owned subsidiary of The Medicines Company, and agreement number HHSO100201600026C with The Medicines Company.
All named authors meet the International Committee of Medical Journal Editors criteria for authorship for the manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
Writing and editorial assistance was provided by Quentin O'Brien of Health and Wellness Partners, LLC, Upper Saddle River, NJ, funded by The Medicines Company.
Christopher M. Rubino and Sujata M. Bhavnani are employees of the Institute for Clinical Pharmacodynamics and grant investigators of The Medicines Company; Jeffrey S. Loutit, Brooke Lohse, Michael N. Dudley, and David C. Griffith are employees of The Medicines Company.
REFERENCES
- 1.Santajit S, Indrawattana N. 2016. Mechanisms of antimicrobial resistance in ESKAPE pathogens. Biomed Res Int 2016:2475067. doi: 10.1155/2016/2475067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 2013. Antibiotic resistance threats in the United States, 2013. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 3.Hecker SJ, Reddy KR, Totrov M, Hirst GC, Lomovskaya O, Griffith DC, King P, Tsivkovski R, Sun D, Sabet M, Tarazi Z, Clifton MC, Atkins K, Raymond A, Potts KT, Abendroth J, Boyer SH, Loutit JS, Morgan EE, Durso S, Dudley MN. 2015. Discovery of a cyclic boronic acid β-lactamase inhibitor (RPX7009) with utility versus class A serine carbapenemases. J Med Chem 58:3682–3692. doi: 10.1021/acs.jmedchem.5b00127. [DOI] [PubMed] [Google Scholar]
- 4.Griffith DC, Loutit JS, Morgan EE, Durso S, Dudley MN. 2016. Phase 1 study of the safety, tolerability, and pharmacokinetics of the β-lactamase inhibitor vaborbactam (RPX7009) in healthy adult subjects. Antimicrob Agents Chemother 60:6326–6332. doi: 10.1128/AAC.00568-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wenzler E, Gotfried MH, Loutit JS, Durso S, Griffith DC, Dudley MN, Rodvold KA. 2015. Meropenem-RPX7009 concentrations in plasma, epithelial lining fluid, and alveolar macrophages of healthy adult subjects. Antimicrob Agents Chemother 59:7232–7239. doi: 10.1128/AAC.01713-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dandekar PK, Maglio D, Sutherland CA, Nightingale CH, Nicolau DP. 2003. Pharmacokinetics of meropenem 0.5 and 2 g every 8 hours as a 3-hour infusion. Pharmacotherapy 23:988–991. doi: 10.1592/phco.23.8.988.32878. [DOI] [PubMed] [Google Scholar]
- 7.Griffith DC, Rubino CM, Loutit JS, Morgan EE, White D, Dudley MN. 2014. A phase 1 study of the safety, tolerability, and pharmacokinetics of a single dose of the beta-lactamase inhibitor RPX7009 alone, meropenem alone, and both in combination (Carbavance) in healthy adult subjects, abstr F-960. Abstr 54th Intersci Conf Antimicrob Agents Chemother, Washington, DC. [Google Scholar]
- 8.Trang M, Griffith DC, Bhavnani SM, Loutit JS, Dudley MN, Ambrose PG, Rubino CM. 2017. Population pharmacokinetics (PPK) of meropenem and vaborbactam in healthy volunteers and infected patients, poster 42. Abstr ASM Microbe, 1 to 5 June 2017, New Orleans, LA. [Google Scholar]
- 9.Bhavnani SM, Trang M, Griffith DC, Lomovskaya O, Hammel JP, Loutit JS, Dudley MN, Ambrose PG, Rubino CM. 2017. Vaborbactam pharmacokinetic-pharmacodynamic (PK-PD) target attainment analyses as support for dose selection in patients with normal renal function and varying degrees of renal impairment, abstr 1852. Abstr IDWeek 2017, 4 to 8 October 2017, San Diego, CA. [Google Scholar]
- 10.The Medicines Company. 2017. Vabomere (meropenem and vaborbactam) prescribing information. The Medicines Company, Parsippany, NJ: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209776lbl.pdf. [Google Scholar]
- 11.AstraZeneca. 2013. Meropenem package insert. AstraZeneca Pharmaceuticals LP, Wilmington DE. [Google Scholar]
- 12.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. 1999. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130:461–470. [DOI] [PubMed] [Google Scholar]
- 13.Cockcroft DW, Gault MH. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 14.National Cancer Institute. 2009. Common terminology criteria for adverse events (CTCAE), version 4.0 U.S. Department of Health and Human Services, NIH, National Cancer Institute, Bethesda, MD: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. [Google Scholar]