FIG 2.
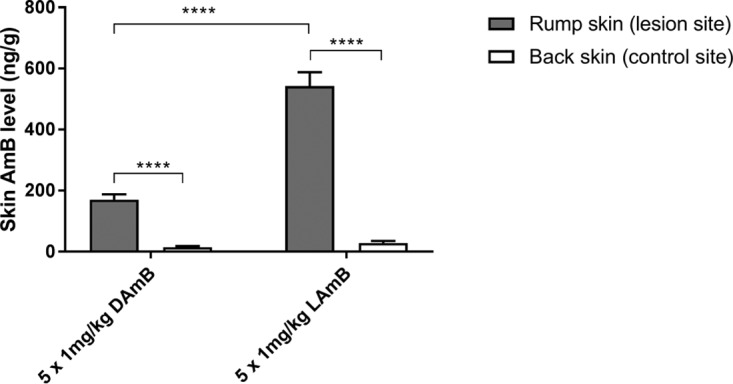
Multiple-dose skin pharmacokinetics of the deoxycholate form of AmB (DAmB) and AmBisome (LAmB). L. major-infected BALB/c mice received intravenous doses of 1 mg/kg on days 0, 2, 4, 6, and 8. On day 10 (48 h after the last dosing), skin samples were collected for amphotericin B (AmB) analysis. The CL lesion was localized on the rump, while the back skin served as a lesion-free, healthy control site. Each point represents the means ± SEM (n = 4 to 5 per group). Differences were analyzed using 1-way ANOVA followed by Tukey's multiple-comparison tests and considered significant at a P value of <0.05 (*) or not significant (ns) if not (****, P < 0.0001).
