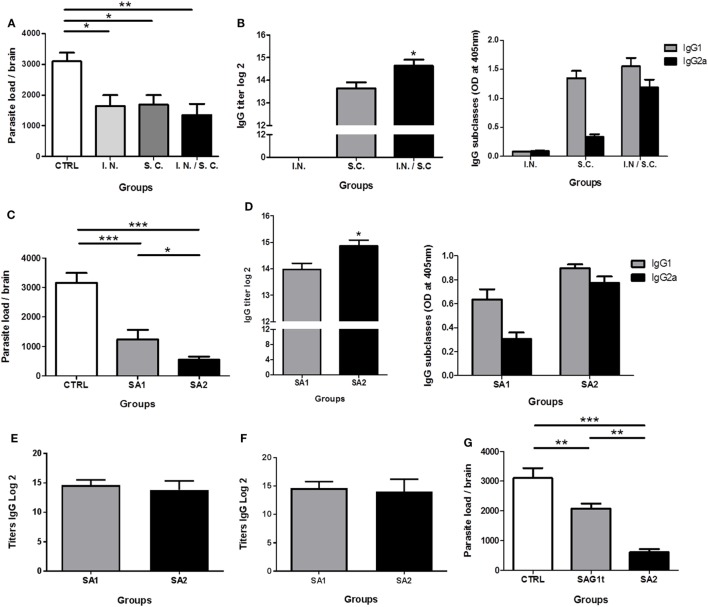
Figure 5.
Evaluation of the protection against chronic toxoplasmosis and humoral response induced following immunization. CBA/J mice (8/group) were primed and boosted twice with SA1, SA2, or SAG1t formulated with polyinosinique-polycytidylique acid (Poly I:C) by different administration routes. Control mice received Poly I:C by the combined routes. (A,B) Mice immunization with SA1 by intranasal, subcutaneous, and combined routes. (C,D) Mice immunization with SA1 and SA2 by combined routes. (D) Mice immunization with SAG1t and SA2 by combined routes. (A,C,G) Protection after vaccination. Protection was evaluated 1 month after challenge by analyzing the cyst load in brain tissue. Results are expressed as the mean ± SEM (n = 8) and represent one of two independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001. Detection of specific anti-SAG1 IgG antibodies and IgG subclasses in sera of immunized mice. Serum samples were tested by ELISA using SAG1t (B,D), P30 protein including the D1 domain and 17 additional amino acids (E) and SA1 (F) as the coating antigen. Results are expressed as the mean ± SEM (n = 8) and represent one of two independent experiments. *P < 0.05.