FIGURE 4.
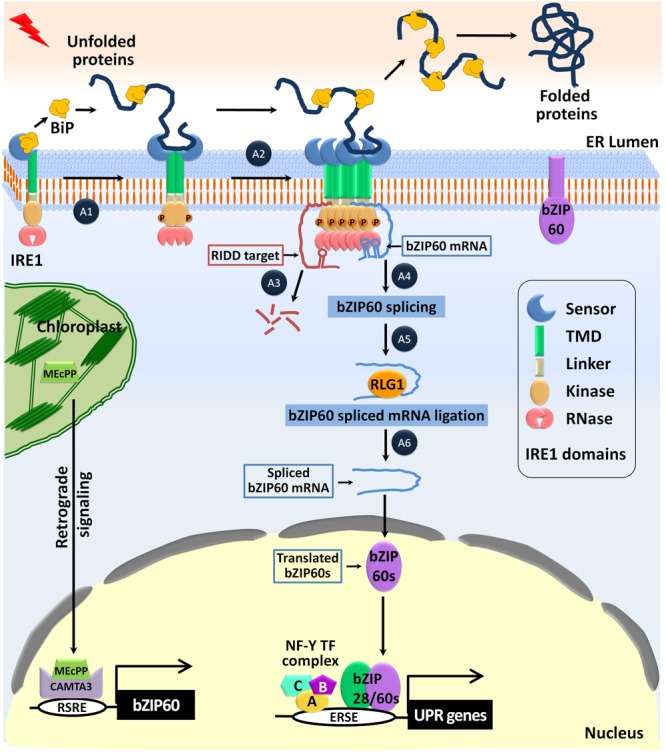
Activation of the IRE1-bZIP60 mediated UPR pathway against ER stress. Under normal conditions, the sensor domain of IRE1 binds to the ER-luminal Binding protein (BiP) and the full-length bZIP60 is anchored in the ER membrane. In response to ER-stress, 2-C-methyl-D-erythritol-2,4-cyclopyrophosphate (MEcPP) is accumulated in the chloroplast and activates the Calmodulin-Binding Transcription Activator 3 (CAMTA3)-TF, which binds to the rapid stress response element (RSRE) and up-regulates bZIP60 expression. A1–A6 indicates the step-wise activation process of IRE1. (A1) In response to ER stress, BiP dissociates from IRE1 to assist the proper folding of the accumulated unfolded proteins. The released IRE1 is dimerized to align its cytosolic kinase domains in such a way that they trans-autophosphorylate each other to activate the RNase function. (A2) The luminal domain of IRE1 binds to the hydrophobic domain of the unfolded proteins and triggers oligomerization and clustering. (A3) The mRNAs encoding the secretory pathway proteins are promiscuously cleaved by the proteins of the regulated IRE1-dependent decay (RIDD) pathway. (A4) The basic linker region of IRE1 mediates specific recruitment and docking of the unspliced bZIP60 mRNA to IRE1 clusters where it is cleaved explicitly at the two conserved sites (CXG| XXG) present in the kissing stem–loop structure. (A5) The tRNA ligase RLG1 catalyzes the ligation of the resulting fragments of the bZIP60 mRNA. (A6) Translation of the spliced bZIP60 mRNA results in the production of the active bZIP60 protein (bZIP60s). It translocates to the nucleus forming the bZIP28-NF-Y-TFs complex. These TF-complexes bind to the ERSE element of the UPR genes and positively regulate the UPR-related downstream gene expression.
