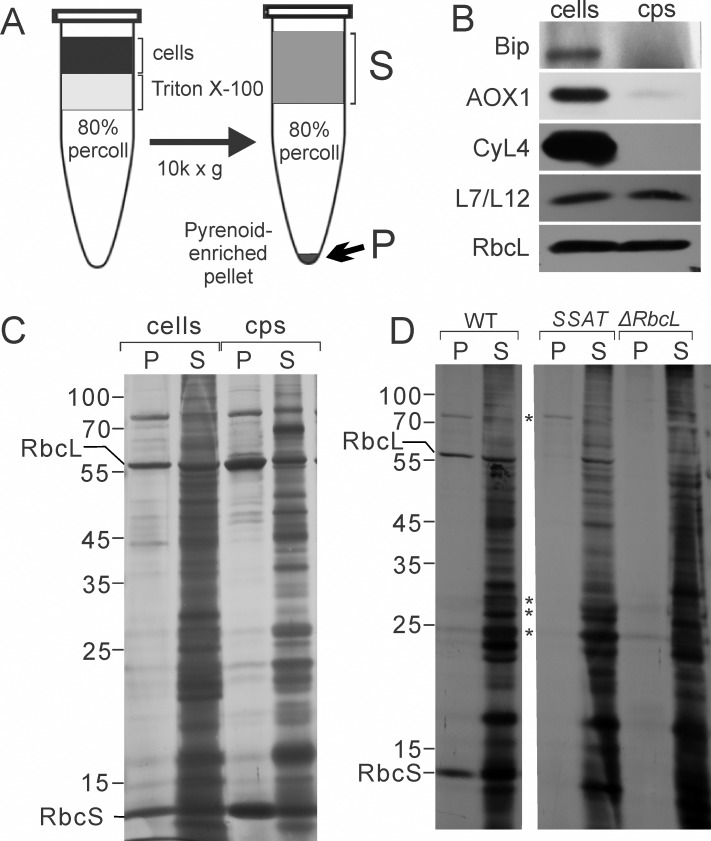
Fig 1. Purification and analysis of pyrenoid preparations.
(A) Pyrenoid-enriched pellet (P) fractions were obtained by solubilizing cells or purified chloroplasts (cps) with Triton X-100 followed by immediate isolation of pyrenoids by centrifugation through a Percoll cushion. Detergent-solubilized material remained in the supernatant (S). Pyrenoids and other material were recovered in the pellet (P). (B) Purification of the chloroplasts from which pyrenoid–enriched fractions were prepared is demonstrated by results of immunoblot analyses comparing extracts of cells and chloroplasts (cps) for the relative levels of marker proteins for ER (Bip), mitochondria (AOX1), cytoplasm (CyL4), and the chloroplast (L7/L12 and RbcL). Samples with 1.0 μg chlorophyll were loaded in each lane. (C and D) Results of SDS-PAGE and silver-staining reveal proteins of the P and S fractions from (C) cells and isolated chloroplasts and (D) WT and the pyrenoid-deficient control strains SSAT and ΔrbcL. (D) Asterisks indicate bands that appear to be contaminants common to P fractions from WT and at least one pyrenoid-deficient mutant. The P and S represent proportional loading of protein isolated from material containing 65 μg chlorophyll.