Scheme 1. Enantioselective Synthesis of the BCDEF Fragment (6S,15R)-4 of (−)-Viridicatumtoxin B [(−)-1] and Its Absolute Configurationa.
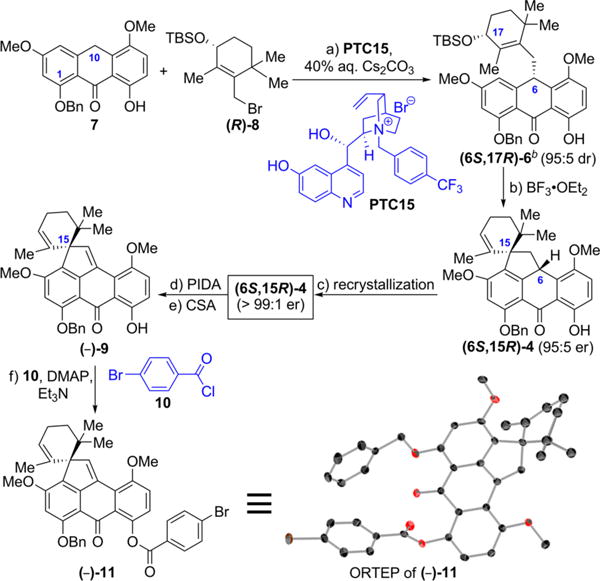
aReagents and conditions: (a) 7 (3.01 g, 8.00 mmol scale), PTC15 (0.5 mol %) 40% aqueous Cs2CO3, (CH2)2Cl2, −30 °C, 10 days, 72%, 95:5 dr; (b) BF3·Et2O (0.05 equiv), CH2Cl2, −78 to 0 °C, 30 min, 74%, 95:5 er; (c) hexanes/CH2Cl2 (50:1), 91%, > 99:1 er; (d) PIDA (1.2 equiv), MeOH/CH2Cl2 (1:1), 0 °C, 30 min, 25 °C, 30 min; (e) CSA (0.07 equiv), CH2Cl2, 0 °C, 5 min, 81% for two steps; (f) 10 (5.0 equiv), DMAP (10 equiv), Et3N (30 equiv), CH2Cl2, 25 °C, 6 h, 95%. bTo avoid confusion, the numbering on (6S,17R)-6, (6S,15R)-4, and (−)-9 in this scheme and Figure 4 is based on the viridicatumtoxin numbering, as opposed to the carbon numbering of compound (10S,14R)-6 (see Table 1), which is the same as (6S,17R)-6, the latter being designated using the anthrone numbering.
