Scheme 2. Total Synthesis of Enantiopure (−)-Viridicatumtoxin B [(−)-1]a.
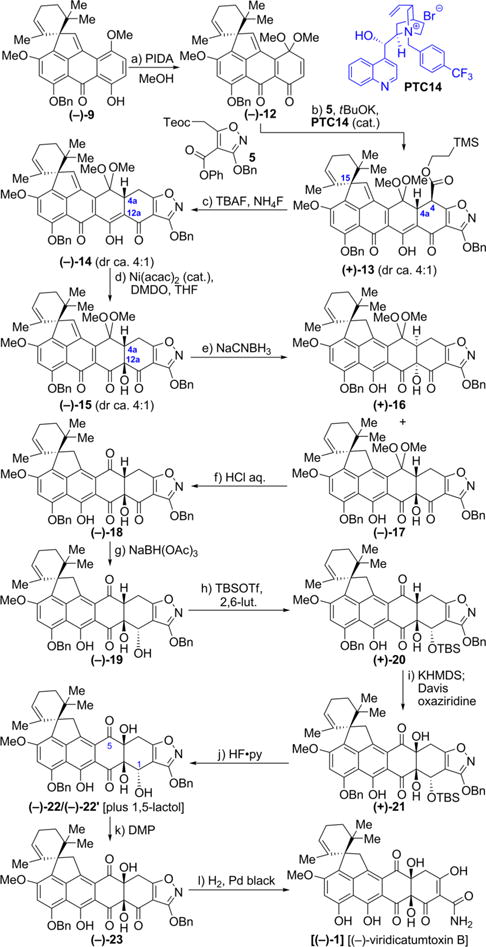
aReagents and conditions: (a) PIDA (1.2 equiv), MeOH/CH2Cl2 (10:1), 25 °C, 1.5 h, 86%; (b) 5 (1.1 equiv), t-BuOK (1.2 equiv), PTC14 (0.01 equiv), toluene, −50 °C, 48 h, 88%, 4:1 dr; (c) TBAF (10 equiv), NH4F (20 equiv), degassed THF, 25 °C, 5 min, 87%, 4:1 dr; (d) [Ni(acac)2] (0.2 equiv), DMDO (3.0 equiv), THF, −78 °C, 3 h, 52% (4:1 dr, 72% brsm), 28% recovered (−)-14; (e) NaCNBH3 (4 equiv), THF, −78 °C, 1.5 h, 48% for (−)-17, 12% for (+)-16, chromatographically separated; (f) 2 N aqueous HCl, THF, 25 °C, 5 h, quant.; (g) NaBH(OAc)3 (1.2 equiv), EtOAc/acetone (1:1), 40 °C, 2 h, 46%; (h) TBSOTf (4 × 10 equiv), 2,6-lutidine (4 × 15 equiv), CH2Cl2, 0 to 25 °C for four times, totaling 2 h, 76%; (i) KHMDS (3.4 equiv), THF, −78 °C, 1 h; then freshly prepared Davis oxaziridine (3.9 equiv), −78 °C, 2 h, 32% of (+)-21 (55% brsm) + 42% recovered (+)-20; (j) HF·pyridine (excess, added at 0 °C in four portions), MeCN, 0 to 55 °C for four times, totaling 20 h, 72% (product 22 exists in equilibrium with its 1,5-lactol isomeric form); (k) DMP solution in CH2Cl2 (0.3 M, 1.8 equiv, added in two portions at 0 °C), (CH2)2Cl2, 0 to 50 °C two times, totaling 2 h, 82%; (l) H2, Pd black (4.9 equiv), 1,4-dioxane/MeOH (1:1), 25 °C, 10 min, 96%.
